Question
Fumaric acid is a naturally occurring dicarboxylic acid.
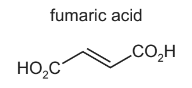
(a) Identify the products of the reaction between fumaric acid and an excess of hot, concentrated, acidified manganate(VII).
(b) Fumaric acid can form addition and condensation polymers.
(i) Draw the repeat unit of the addition polymer poly(fumaric acid).
(ii) Draw the repeat unit of the polyester formed when fumaric acid reacts with ethane-1,2-diol,
(CH2OH)2.
The ester bond should be shown fully displayed.
(iii) Explain why polyesters normally biodegrade more readily than polyalkenes.
(c) Fumaric acid reacts with cold, dilute, acidified manganate(VII) to form compound P.
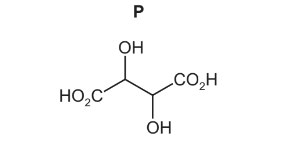
Only three stereoisomers of P exist. One of the stereoisomers is shown.
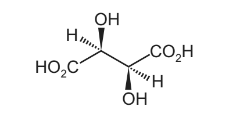
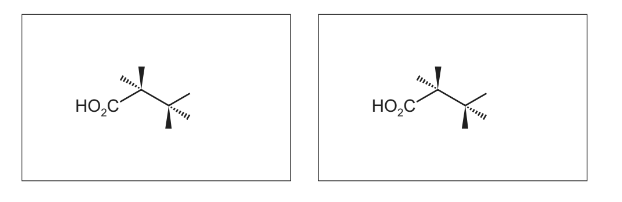
Complete the three-dimensional diagrams in the boxes to show the other two stereoisomers of P.
(d) The enzyme fumarase catalyses the reaction of fumarate ions, C4H2O42-, with water to form malate ions, C4H4O52-.
\(C_{4}H_{2}O_{4}^{2-} + H_{2}O \rightleftharpoons C_{4}H_{4}O_{5}^{2-}\)
Describe, with the aid of a suitably labelled diagram, how an enzyme such as fumarase can catalyse a reaction.
Answer/Explanation
Answer: (a) CO2 and H2O / in words
(b)(i) 
(b)(ii) 
(b)(iii) C—C bonds are non-polar / polyalkenes cannot be hydrolysed
OR polyesters / they can be broken down by hydrolysis
(c) 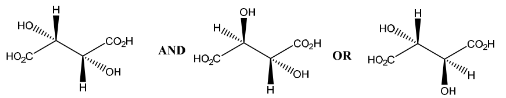
(d) 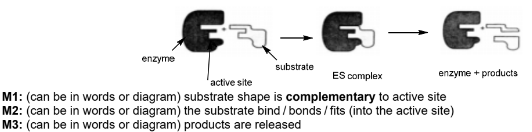
Question
(a) Methyl 2-cyanoprop-2-enoate, W, is the major component of Super Glue, a rapid-setting adhesive.
As the adhesive sets, the monomer W polymerises.
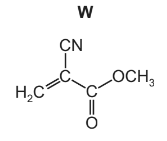
(i) Draw a section of the polymer showing two repeat units.
(ii) Name the type of polymerisation occurring.
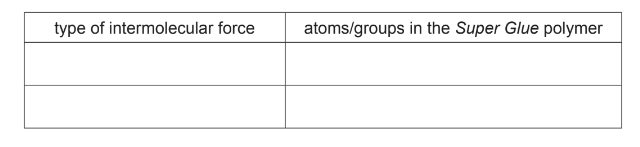
(iii) Suggest two types of intermolecular force that could occur between the Super Glue
polymer and the objects glued together. For each type of intermolecular force, refer to the
atoms/groups in the Super Glue polymer involved in the attraction.
(b) W can be synthesised in three steps, starting from 2-oxopropanoic acid, X.
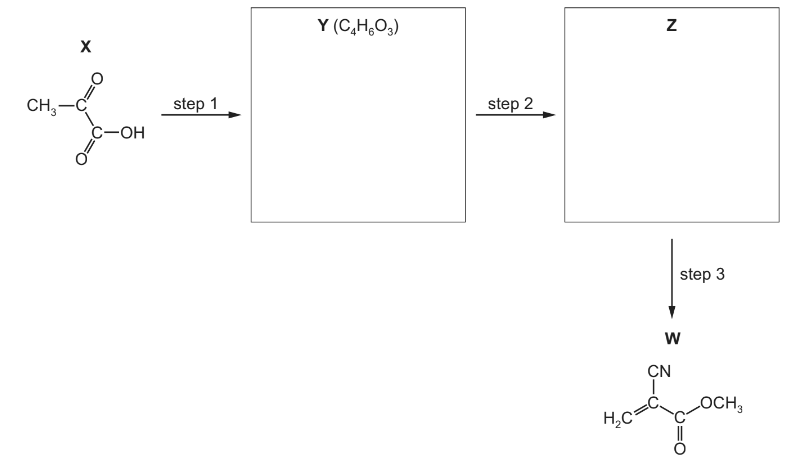
(i) Suggest the identities of compounds Y and Z by drawing their structures in the boxes.
(ii) Suggest suitable reagents and conditions for each of the steps 1–3.
step 1 _____________________________________
step 2 _____________________________________
step 3 _____________________________________
Answer/Explanation
Answer: (a)(i)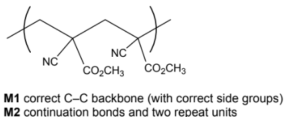
(a)(ii) addition
(a)(iii) Any two of:
permanent dipole (attraction): C, N, O, OR CO, CN, CO2CH3, OCH3
H-bonding: N, O OR CO, CN
London/van der Waals: N, C, H, O OR CH3, CN, CO2CH3, C–C chains
(b)(i) Y CH3COCO2CH3
Z CH3C(OH)(CN)CO2CH3
(b)(ii) M1/M2 step 1: CH3OH and (conc) H2SO4 + heat M3 step 2: HCN + NaCN catalyst
M4 step 3: T > 100oC / heat with Al2O3 (or heat with c. H2SO4)