Question
(a) Describe what is meant by a racemic mixture.
(b) Asparagine is an amino acid that contains a chiral carbon atom and displays stereoisomerism.
Separate samples of asparagine are dissolved in \(CDCl_3\) and analysed using carbon-13 and proton (\(^1H\)) NMR spectroscopy.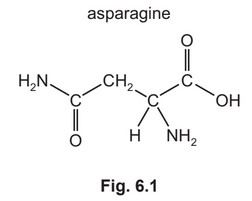
Predict the number of peaks seen in the carbon-13 and proton (\(^1H\)) NMR spectra of asparagine.
(c) The isoelectric point of asparagine, asn, is at pH 5.4.
(i) Describe the meaning of the term isoelectric point.
(ii) Draw the structure of asparagine at pH 1.0.
(d) Asparagine can polymerise to form poly(asparagine).
Draw the structure of poly(asparagine), showing two repeat units. The peptide linkage should be shown displayed.
(e) The isoelectric point of lysine, lys, is at pH 9.8.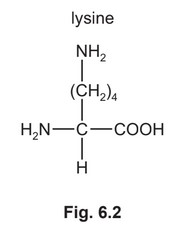
A mixture of the dipeptide lys-asn and its two constituent amino acids, asparagine and lysine, is analysed by electrophoresis using a buffer at pH 5.0. The results obtained are shown in
Fig. 6.3.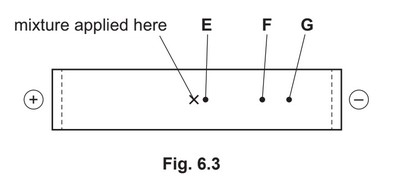
Suggest identities for the species responsible for spots E, F and G. Explain your answers.
(f) Thin-layer and gas-liquid chromatography can be used to analyse mixtures of substances.
Each type of chromatography makes use of a stationary phase and a mobile phase.
(i) Complete Table 6.1 with an example of each of these.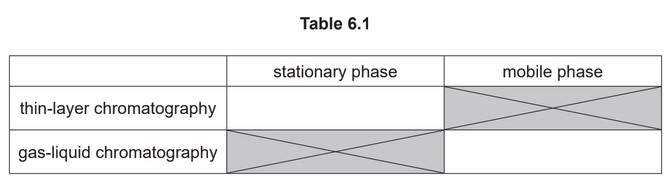
(ii) An unknown amino acid is analysed using thin-layer chromatography. Two chromatographs of the unknown amino acid and four reference amino acids, P, Q, R and S, are obtained using two different solvents.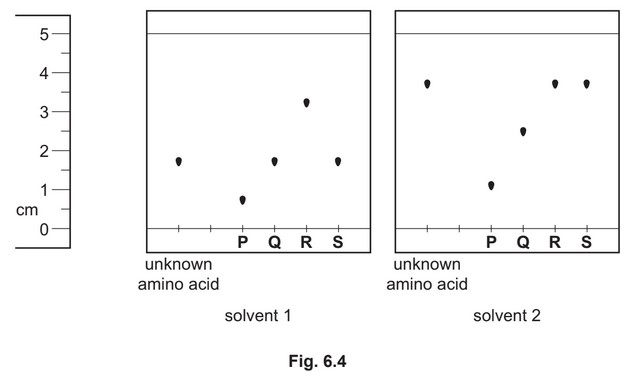
Identify the unknown amino acid. Justify your answer.
(g) A mixture containing three organic compounds is analysed by gas chromatography and mass spectrometry. The gas chromatogram is shown.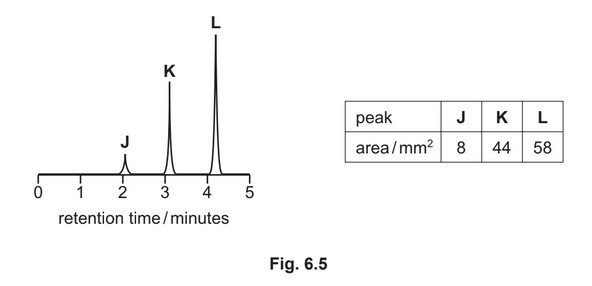
The area underneath each peak is proportional to the mass of the respective compound in the
mixture.
The concentration of K in the mixture is \(5.52 × 10^{–2} gdm^{–3}\).
Calculate the concentration, in moldm–3, of compound L in the mixture.
[\(M_r\): L, 116]
concentration of L = ………………………… \(moldm^{–3}\)
Answer/Explanation
Answer:
(a) a mixture containing equal amounts of each optical isomer
(b) 
(c) (i) the pH at which an amino acid exists as a zwitterion
OR
the pH at which an amino acid has no overall charge
(ii) 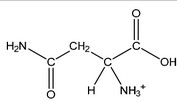
(d) 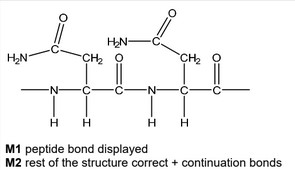
(e) 
M1 table correctly completed
M2 Lys and Lys-Asn are positively charged OR Asn is (nearly) uncharged
M3 LysAsn has the highest \(M_r\)
(f) (i) aluminum oxide / silica (on solid support) AND inert gas / named inert gas e.g. \(N_2\)
(ii) S AND
\(R_f\) is the same as the unknown amino acid in both solvents
(g) mass of L = 58 / 44 * 5.52 \times 10^{–2} = 7.28
\times 10^{–2}\) g
conc. of L = 7.28 \times 10^{–2} / 116 = 6.27 \times 10^{–4} (mol dm^{–3}) min 2sf\)
Question
Lidocaine is used as an anaesthetic. A synthesis of lidocaine is shown in Fig. 6.1.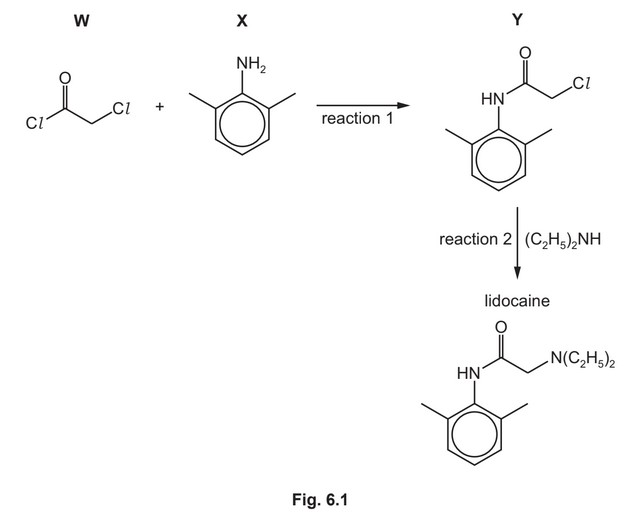
(a) W can be formed by reacting \(HOCH_2COOH\) with an excess of \(SOCl_2\).
Write an equation for this reaction.
(b) After W and X have reacted together, an excess of \(CH_3COONa(aq)\) is added to the reaction
mixture.
Suggest why.
(c) The reaction of W with X, reaction 1, follows an addition–elimination mechanism.
Complete the mechanism for the reaction of W with X.
Include all relevant curly arrows, lone pairs of electrons, charges and partial charges.
Use \(Ar–NH_2\) to represent X.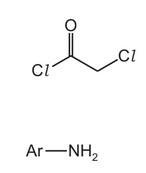
(d) \((C_2H_5)_2NH\) reacts with Y in reaction 2.
Explain why \((C_2H_5)_2NH\) can act as a nucleophile.
(e) The purity of lidocaine can be checked using thin-layer chromatography.
Ethyl ethanoate is used as the solvent.
The \(R_f\) values of X and lidocaine are given in Table 6.1.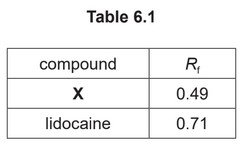
(i) Identify the substances used as the mobile and stationary phases in this thin‐layer chromatography experiment.
mobile phase ……………………………………………………………………………………………………….
stationary phase …………………………………………………………………………………………………..
(ii) Describe how an \(R_f\) value can be calculated.
(iii) Suggest why the \(R_f\) value for X is less than that for lidocaine.
(f) The proton \((^1H)\) NMR spectrum of lidocaine is shown in Fig. 6.2.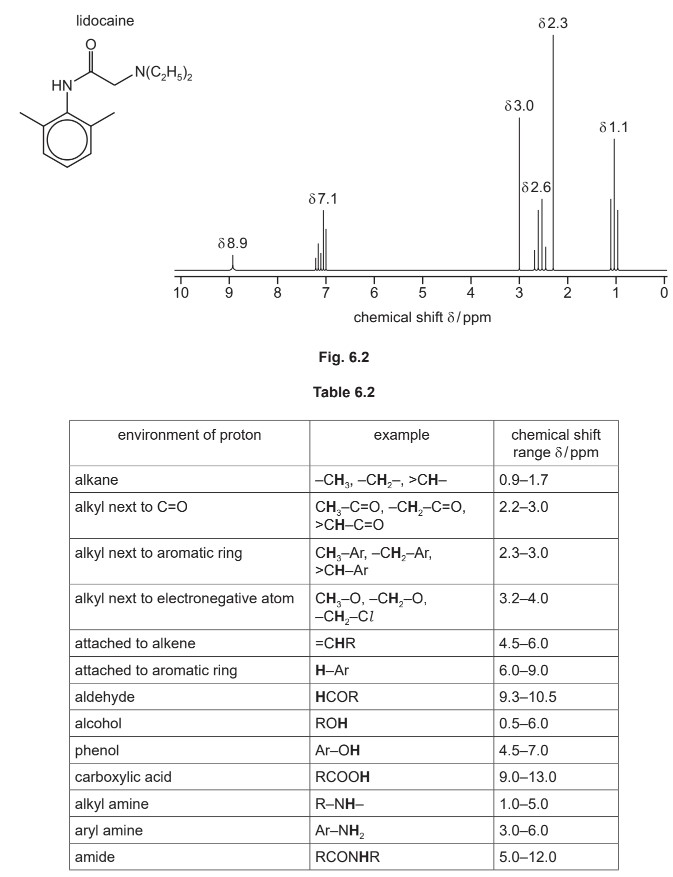
(i) Name the splitting patterns at δ2.6 and δ1.1.
δ2.6 ……………………………………………………. δ1.1 …………………………………………………….
(ii) The relative peak area of the peaks at δ3.0 and δ2.3 is 1:3 respectively.
Identify the protons in the \(^1H\) NMR spectrum of lidocaine that are responsible for the peaks
at the following chemical shift values.
δ7.1 ……………………………………………………………………………………………………………………
δ3.0 ……………………………………………………………………………………………………………………
δ2.3 ……………………………………………………………………………………………………………………
(iii) Predict the number of peaks in the carbon‐13 \((^{13}C)\) NMR spectrum of lidocaine.
Answer/Explanation
Answer:
(a)\(HOCH_2COOH + 2SOCl_2 \rightarrow ClCH_2COCl + 2SO_2 + 2HCI\)
(b) to remove / neutralise excess \(H^+\)/ acid produced
OR to react with any acidic by-products / HCl /\(SO_2\)
OR to react with any unreacted W
(c) 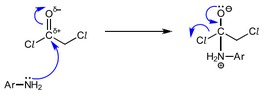
M1: curly arrow from lone pair on :\(NH_2\) to carbonyl
\(C^{(δ+)}=O\)
M2: correct dipole on \(^{δ+}C=O^{δ–}\) AND curly arrow from bond C=O to \(O^{(δ–)}\)
M3: correct structure of the intermediate (inc. charges)
M4: curly arrow from lone pair on :O– to C=O AND curly arrow from C—Cl to Cl
(d) N / nitrogen can donate its lone pair / LP / pair of electrons
(e) (i) mobile = ethyl ethanoate
stationary = \(SiO_2\) / silica or \(Al_2O_3\) / alumina
(ii) \(R_f\) = distance travelled by solute / substance / compound / component
÷ distance travelled by solvent (front)
(iii) X is more attracted / more affinity / adsorbed more to the stationary phase
OR lidocaine dissolves better in the solvent ORA
(f) (i) quartet AND triplet
(ii) • δ 7.1 = attached to aromatic ring / H—Ar
• δ 3.0 = alkyl next to \(C=O / —CH_{(2)}–C=O\)
• δ 2.3 = alkyl next to aromatic ring / \(H_{(3)}C–Ar\)
All three correct for two marks
(iii) 9 (nine)