Question
(a) Benzene reacts with bromine in the presence of an aluminium bromide catalyst, $\mathrm{AlBr}_3$, to form bromobenzene. This is a substitution reaction. No addition reaction takes place.
(i) Explain why no addition reaction takes place. [1]
$\mathrm{AlBr}_3$ reacts with bromine to generate an electrophile, $\mathrm{Br}^{+}$.
(ii) Draw the mechanism of the reaction between benzene and $\mathrm{Br}^{+}$ions. Include all relevant arrows and charges.[3]
(iii) Write an equation to show how the $\mathrm{AlBr}_3$ catalyst is reformed.[1]
(b) Suggest why bromination of phenol occurs more readily than bromination of benzene.[2]
(c) (i) There are four different carbocations with the same formula, $\mathrm{C}_4 \mathrm{H}_9^{+}$. One structure is given in the table.
Suggest the structural formulae of the three other carbocations.
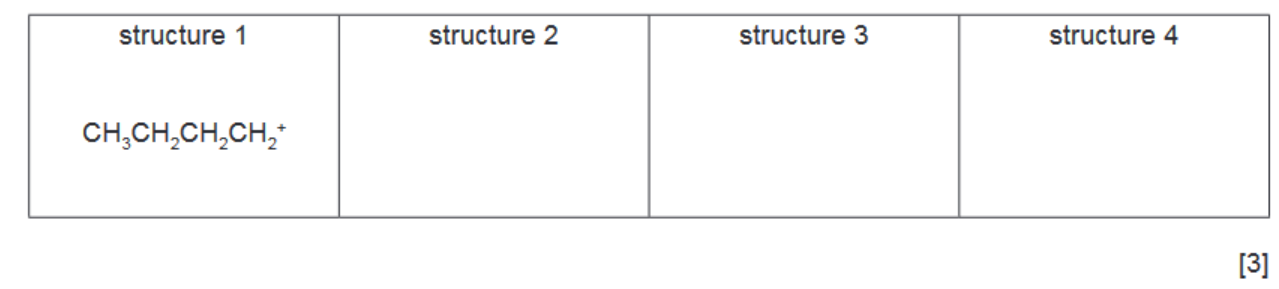 [3]
[3]
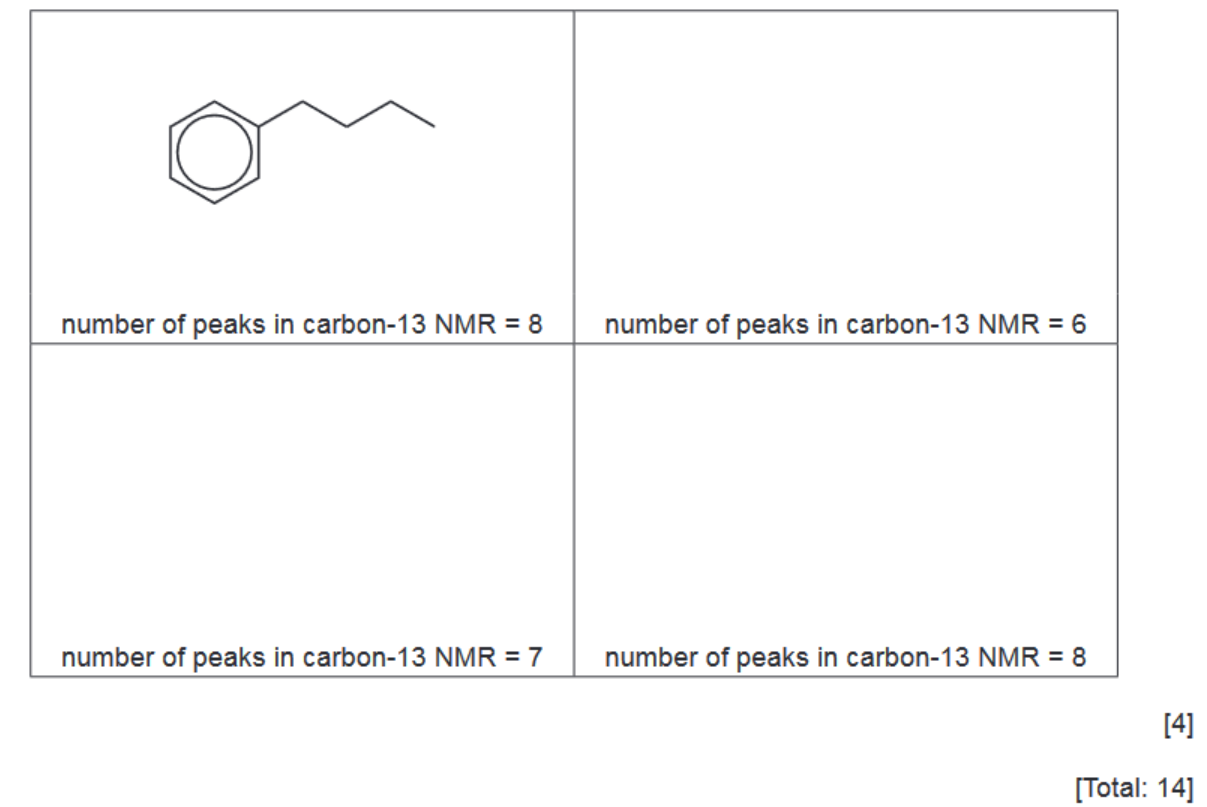
(ii) Benzene reacts with each of these carbocations in separate Friedel-Crafts alkylation reactions.
In each reaction an organic compound with formula $\mathrm{C}_{10} \mathrm{H}_{14}$ is formed. The number of peaks observed in the carbon-13 NMR spectrum of each compound is given.
Suggest the structures for the three other compounds.
▶️Answer/Explanation
Ans:
(a)(i) The substitution product is stabilised by delocalisation of (6)π-electrons
OR
The addition product is not stabilised by delocalisation of (6)π-electrons [1]
(a)(ii)
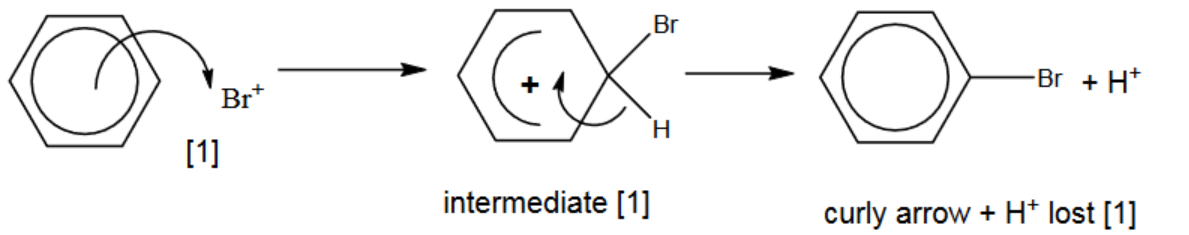
- first curly arrow
- intermediate
- $2^{\text {nd }}$ curly arrow, product and $\mathrm{H}^{+}$formed / lost
$5(\mathrm{a})$ (iii) $\quad \mathrm{AlBr}_4^{-}+\mathrm{H}^{+} \rightarrow \mathrm{AlBr}_3+\mathrm{HBr}$
5(b) lone pair of oxygen is delocalised into the ring
any one from:
• phenol has a higher electron density in the ring
• phenol can polarise/induce a dipole in $ Br_{2}$
5(c)(i) $\quad \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}^{+} \mathrm{CH}_3 \quad\left(\mathrm{CH}_3\right)_2 \mathrm{CHCH}_2{ }^{+} \quad\left(\mathrm{CH}_3\right)_3 \mathrm{C}^{+} \quad$ Each correct structure $=1$ mark
(c)(ii)
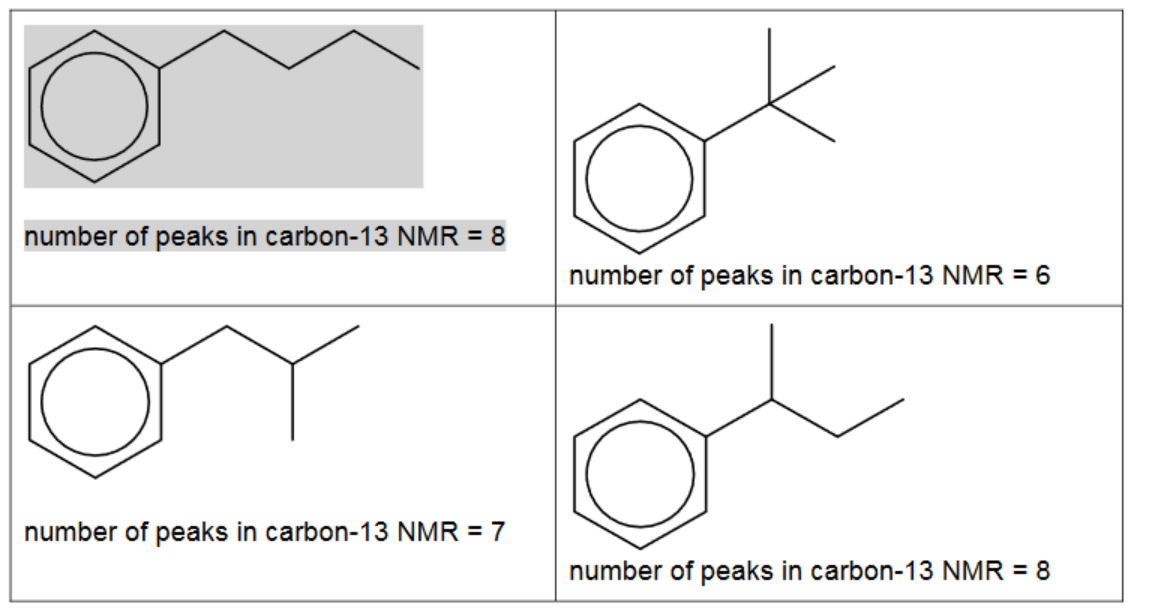
Two correct organic products = 1 mark
three correct organic products = 2 marks
all products linked correctly to NMR = 2 marks
Question
(a) Ethanedioic acid, $\mathrm{C}_2 \mathrm{O}_4 \mathrm{H}_2$, occurs in many vegetables. The amount that occurs in spinach can be estimated as follows.
- $\quad 40.0 \mathrm{~g}$ of spinach leaves are crushed and mixed with distilled water, using a mortar and pestle.
- The mixture is filtered, and the leaves are washed with a little more water.
- The combined filtrate and washings are made up to $100.0 \mathrm{~cm}^3$ with water.
- A $25.0 \mathrm{~cm}^3$ portion of the resulting solution is added to a conical flask, along with an excess of dilute sulfuric acid.
- The acidified solution is warmed, and then titrated with $0.0200 \mathrm{moldm}^{-3} \mathrm{KMnO}_4$.
The equation for the reaction between ethanedioic acid and acidified manganate(VII) ions is shown.
$
2 \mathrm{MnO}_4^{-}+6 \mathrm{H}^{+}+5 \mathrm{C}_2 \mathrm{O}_4 \mathrm{H}_2 \rightarrow 2 \mathrm{Mn}^{2+}+10 \mathrm{CO}_2+8 \mathrm{H}_2 \mathrm{O}
$
In the titration, $15.20 \mathrm{~cm}^3$ of $\mathrm{KMnO}_4$ was required to reach the end-point.
Calculate the percentage by mass of ethanedioic acid in the spinach leaves.
percentage of ethanedioic acid = ……………………….. % [3]
(b) Ethanedioic acid can be converted into ethanedioyl chloride:
$
\mathrm{HO}_2 \mathrm{CCO}_2 \mathrm{H} \rightarrow \mathrm{C} l \mathrm{OCCOC} l
$
(i) State a suitable reagent for this reaction.[1]
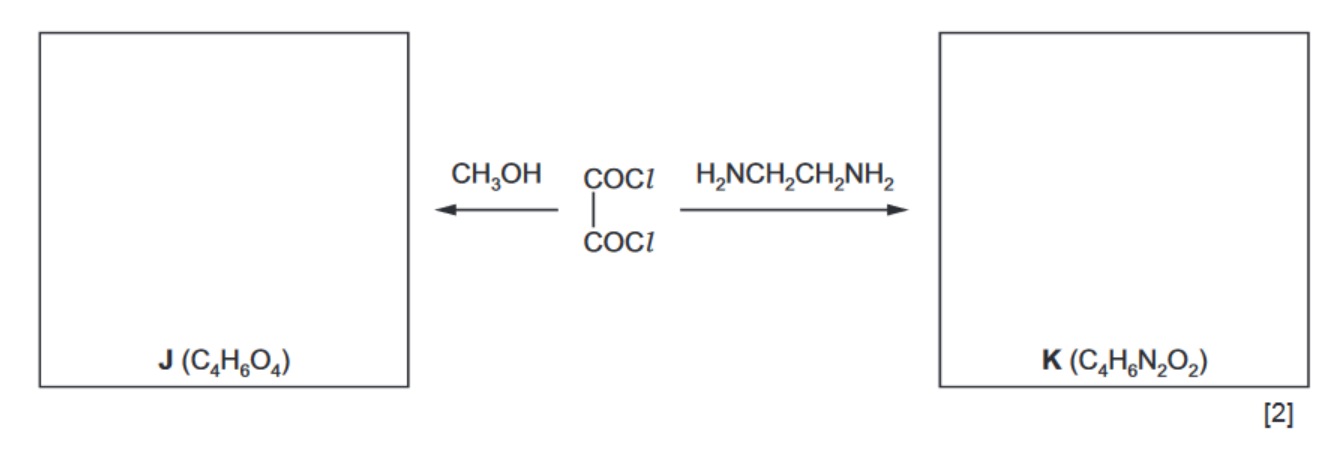
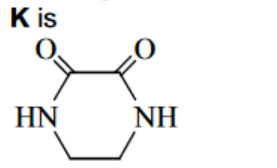
(ii) For the reactions of ethanedioyl chloride below, suggest the structures of compounds $\mathbf{J}$ and $\mathbf{K}$ and draw them in the boxes.
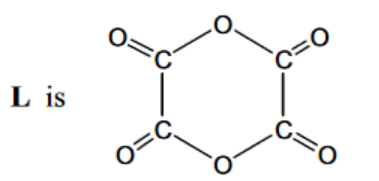
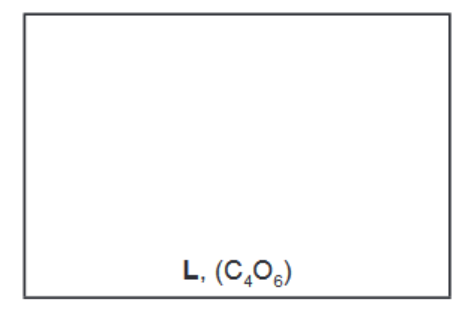
(c) When ethanedioyl chloride is reacted with silver ethanedioate, $\mathrm{AgO}_2 \mathrm{CCO}_2 \mathrm{Ag}$, in ethoxyethane at $-30^{\circ} \mathrm{C}$, an oxide of carbon, $L$, is formed. The molecule of $L$ has no overall dipole and has molecular formula $\mathrm{C}_4 \mathrm{O}_6$.
The carbon-13 NMR spectrum of a solution of $\mathrm{L}$ in ethoxyethane, $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OCH}_2 \mathrm{CH}_3$, is shown below.
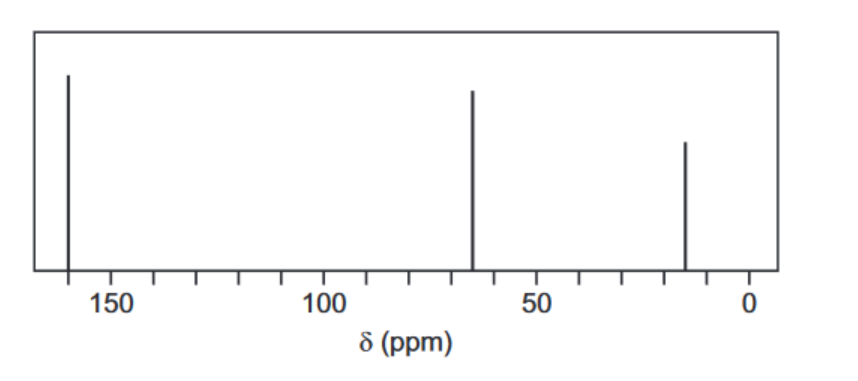
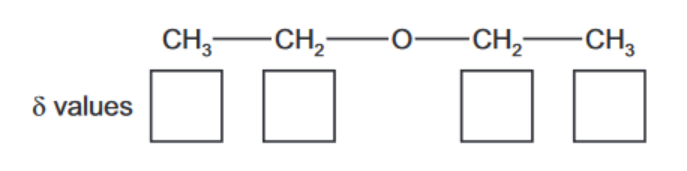
(i) Use the Data Booklet to state in the boxes below the $\delta$ values for the peaks in the spectrum which are due to the carbon atoms in ethoxyethane.
(i) Explain what the rest of the carbon-13 NMR spectrum indicates about the structure of L. ……………………………………………………………………………………………………………………… [1]
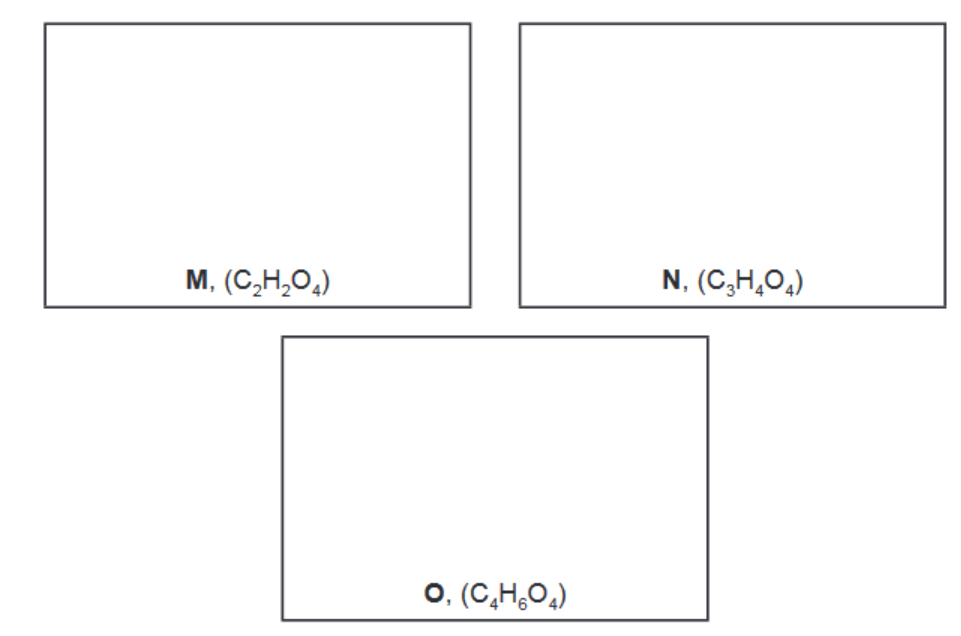
(d) When pure $\mathrm{L}$ is reacted with an excess of $\mathrm{CH}_3 \mathrm{OH}$, a mixture of three compounds is formed.
$\begin{gathered}\mathbf{L} \\ \left(\mathrm{C}_4 \mathrm{O}_6\right)\end{gathered}$ and $\mathrm{CH}_3 \mathrm{OH} \rightarrow \underset{\left(\mathrm{C}_2 \mathrm{H}_2 \mathrm{O}_4\right)}{\rightarrow} \quad \begin{gathered}\mathbf{M} \\ \left(\mathrm{C}_3 \mathrm{H}_4 \mathrm{O}_4\right)\end{gathered}$ and $\begin{gathered}\mathbf{O} \\ \left(\mathrm{C}_4 \mathrm{H}_6 \mathrm{O}_4\right)\end{gathered}$
M is formed as one of the products when either N or O is heated with aqueous acid.
The table gives information of the peaks recorded in the carbon-13 NMR spectra of M, N and O
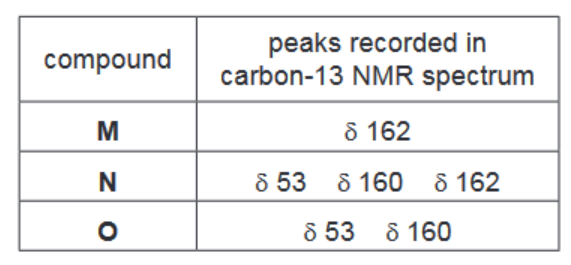
i) Suggest the structures of M, N and O
ii) Suggest a structure for L that fits all the data given in (c) and (d).
 [1] [Total: 13]
[1] [Total: 13]
▶️Answer/Explanation
Ans:
(a)
$
\begin{aligned}
& \mathrm{n}\left(\mathrm{MnO}_4{ }^{-}\right)=0.02 \times 15.2 / 1000=3.04 \times 10^{-4} \mathrm{~mol} \\
& \mathrm{n}\left(\mathrm{C}_2 \mathrm{O}_4 \mathrm{H}_2\right)=3.04 \times 10^{-4} \times 5 / 2=7.6 \times 10^{-4}\left(\text { in } 25 \mathrm{~cm}^3\right)=3.04 \times 10^{-3} \mathrm{~mol} \text { in } 100 \mathrm{~cm}^3 \\
& \mathrm{M}_{\mathrm{r}}=24+64+2=90 \\
& \text { mass of } \mathrm{C}_2 \mathrm{O}_4 \mathrm{H}_2=3.04 \times 10^{-3} \times 90 \\
& =0.2736 \mathrm{~g}(0.274) \\
& \text { percentage }=0.2736 \times 100 / 40=0.68 \%
\end{aligned}
$
(b) (i) $\mathrm{SOCl}_2$ or $\mathrm{PCl}_5$ or $\mathrm{PCl}_3$
(ii) $\mathbf{J}$ is $\mathrm{CH}_3 \mathrm{OCO}-\mathrm{COOCH}_3$
(c) (i) $\quad \begin{aligned} & \mathrm{CH}_3 \text { at } \delta 15 \\ & \mathrm{CH}_2 \mathrm{O} \text { at } \delta 65\end{aligned}$
(ii) Only one peak, so only one type / environment of C atom
(d) (i) $\mathbf{M}$ is $\mathrm{HO}_2 \mathrm{C}-\mathrm{CO}_2 \mathrm{H}$
$\mathbf{N}$ is $\mathrm{CH}_3 \mathrm{OCO}-\mathrm{CO}_2 \mathrm{H}$
O is $\mathrm{CH}_3 \mathrm{OCO}-\mathrm{COOCH}_3$
(ii)