Question
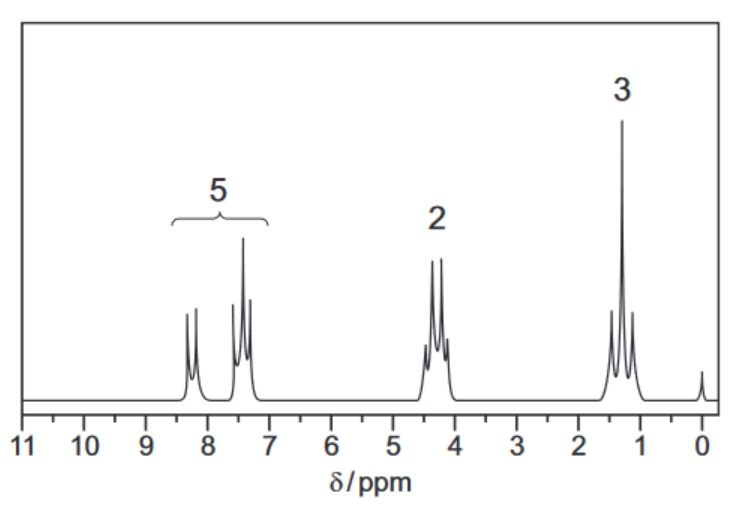
The proton NMR spectrum of compound $\mathbf{E}$ in the solvent $\mathrm{CDC} l_3$ is shown. The molecular formula of compound $\mathbf{E}$ is $\mathrm{C}_9 \mathrm{H}_{10} \mathrm{O}_2$.
(a) Explain why $\mathrm{CDCl}_3$ is used as a solvent instead of $\mathrm{CHCl}_3$.[1]
(b) Explain why TMS is added to give the small peak at chemical shift $\delta=0$.[1]
(c) Compound $\mathbf{E}$ is hydrolysed by hot $\mathrm{NaOH}(\mathrm{aq})$, giving two organic products only. One of these products is ethanol.
Name the functional group in compound $\mathbf{E}$ that is hydrolysed by hot $\mathrm{NaOH}(\mathrm{aq})$.[1]
(d) (i) Describe and explain the splitting patterns of the peaks at $\delta=1.4$ and $\delta=4.3$.
splitting pattern at $\delta=1.4$
reason for splitting pattern at $\delta=1.4$
splitting pattern at $\delta=4.3$
reason for splitting pattern at $\delta=4.3$[2]
(ii) Each molecule of compound $\mathbf{E}$ contains five protons which give rise to the peaks between $\delta=7.0$ and $\delta=8.5$.
Identify the functional group in compound $\mathbf{E}$ which contains these protons.[1]
(iii) Give the structural formula of compound $\mathbf{E}$.[1]
(e) The mass spectrum of compound $\mathbf{E}$ includes fragment ions with m/e values of 29 and 77 . Give the formulae of these fragment ions.
fragment ion with $m / e=29$
fragment ion with $m / e=77$[5][Total: 9]
▶️Answer/Explanation
Ans:
(a) (because $\mathrm{CDC} l_3 /$ it) does not give a peak [1] OR because $\mathrm{CHCl}_3$ does give a peak
(b) as a standard / reference for (chemical shift measurements) [1]
(c) ester [1] 1
9(d)(i) • (δ = 1.4) triplet
• (δ = 1.4) two H on neighbouring C atom
• (δ = 4.3) quartet / quadruplet
• (δ = 4.3) three H on neighbouring C atom
mark as $\bullet \checkmark \bullet \checkmark[2]$
9(d)(ii) aryl group / arene / phenyl [1]
(d)(iii)
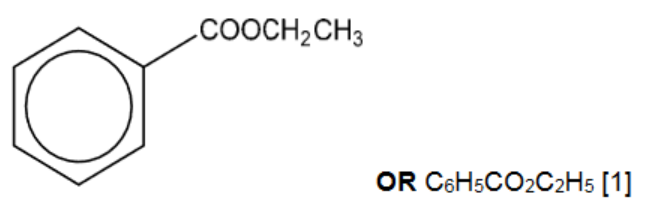
(e)$\mathrm{CH}_3 \mathrm{CH}_2^{+} / \mathrm{C}_2 \mathrm{H}_5^{+}[1]$
$\mathrm{C}_6 \mathrm{H}_5^{+}[1]$
Question
(a) Describe what is meant by a racemic mixture.
(b) Asparagine is an amino acid that contains a chiral carbon atom and displays stereoisomerism.
Separate samples of asparagine are dissolved in \(CDCl_3\) and analysed using carbon-13 and proton (\(^1H\)) NMR spectroscopy.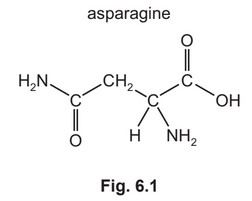
Predict the number of peaks seen in the carbon-13 and proton (\(^1H\)) NMR spectra of asparagine.
(c) The isoelectric point of asparagine, asn, is at pH 5.4.
(i) Describe the meaning of the term isoelectric point.
(ii) Draw the structure of asparagine at pH 1.0.
(d) Asparagine can polymerise to form poly(asparagine).
Draw the structure of poly(asparagine), showing two repeat units. The peptide linkage should be shown displayed.
(e) The isoelectric point of lysine, lys, is at pH 9.8.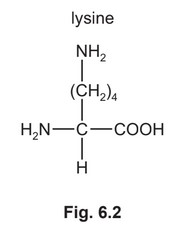
A mixture of the dipeptide lys-asn and its two constituent amino acids, asparagine and lysine, is analysed by electrophoresis using a buffer at pH 5.0. The results obtained are shown in
Fig. 6.3.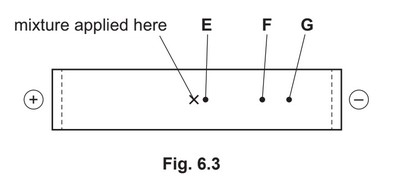
Suggest identities for the species responsible for spots E, F and G. Explain your answers.
(f) Thin-layer and gas-liquid chromatography can be used to analyse mixtures of substances.
Each type of chromatography makes use of a stationary phase and a mobile phase.
(i) Complete Table 6.1 with an example of each of these.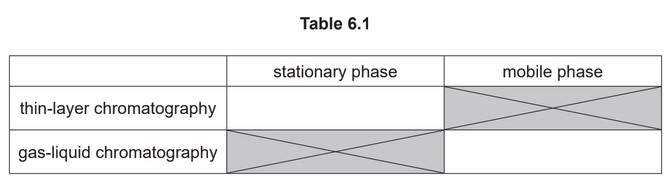
(ii) An unknown amino acid is analysed using thin-layer chromatography. Two chromatographs
of the unknown amino acid and four reference amino acids, P, Q, R and S, are obtained
using two different solvents.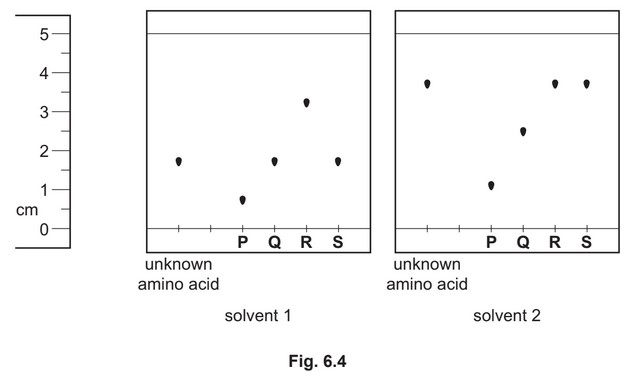
Identify the unknown amino acid. Justify your answer.
(g) A mixture containing three organic compounds is analysed by gas chromatography and mass spectrometry. The gas chromatogram is shown.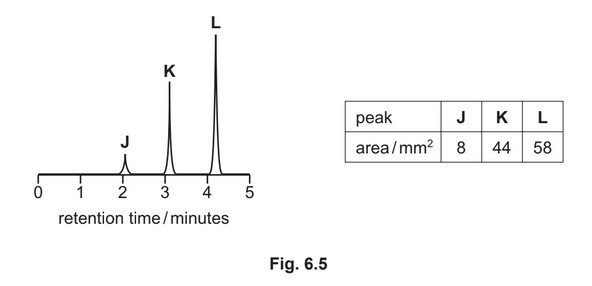
The area underneath each peak is proportional to the mass of the respective compound in the
mixture.
The concentration of K in the mixture is \(5.52 × 10^{–2} gdm^{–3}\).
Calculate the concentration, in moldm–3, of compound L in the mixture.
[\(M_r\): L, 116]
concentration of L = ………………………… \(moldm^{–3}\)
Answer/Explanation
Answer:
(a) a mixture containing equal amounts of each optical isomer
(b) 
(c) (i) the pH at which an amino acid exists as a zwitterion
OR
the pH at which an amino acid has no overall charge
(ii) 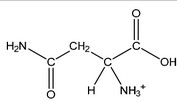
(d) 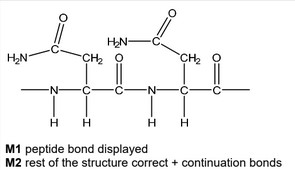
(e) 
M1 table correctly completed
M2 Lys and Lys-Asn are positively charged OR Asn is (nearly) uncharged
M3 LysAsn has the highest \(M_r\)
(f) (i) aluminum oxide / silica (on solid support) AND inert gas / named inert gas e.g. \(N_2\)
(ii) S AND
\(R_f\) is the same as the unknown amino acid in both solvents
(g) mass of L = 58 / 44 * 5.52 \times 10^{–2} = 7.28
\times 10^{–2}\) g
conc. of L = 7.28 \times 10^{–2} / 116 = 6.27 \times 10^{–4} (mol dm^{–3}) min 2sf\)