Question:
Many gases do not obey the general gas equation at high pressures. Why is this?
1 At higher pressures the molecules have more energy.
2 At higher pressures the volume of the molecules is a larger proportion of the total volume.
3 At higher pressures the molecules experience greater intermolecular forces
▶️Answer/Explanation
Ans:C
Question:
A solution contains 0.25 g of sulfur dioxide in \(1.00~dm^{3}\) of water. Which volume of sulfur dioxide, measured at \(50^{\circ}C\) and a pressure of \(1~x~10^{5}Pa\) , must be added to \(1.00dm^{3}\) of water to produce this solution?
A $\quad 0.0162 \mathrm{~cm}^3$
B $\quad 0.105 \mathrm{~cm}^3$
C $\quad 16.2 \mathrm{~cm}^3$
D $\quad 105 \mathrm{~cm}^3$
▶️Answer/Explanation
Ans:D
Question
\(10cm^{3}\) of ethane is burned in \(45cm_{3}\) of oxygen at a pressure of 101kPa and a temperature of 200 °C. Complete combustion takes place.
What is the total volume of gas present when the reaction is complete, measured under the same conditions?
A \(30cm^{3}\) B \(50cm^{3}\) C \(55cm^{3}\) D \(60cm^{3}\)
▶️Answer/Explanation
Ans:D
Question
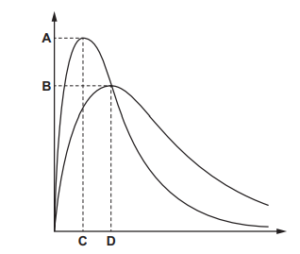
The diagram shows the Boltzmann energy distribution curves for molecules of a sample of a gas at two different temperatures.
Which letter on the axes represents the most probable energy for molecules of the same sample of gas at the lower temperature?
▶️Answer/Explanation
Ans:C
Question
A sample of argon gas has a mass of 0.20 g, at a pressure of 100 000Pa and a temperature of 12 °C
Which volume does the gas occupy?
A \(1.2~x~10^{-4}cm^{3}\)
B \(5.0cm^{3}\)
C \(59cm^{3}\)
D \(119cm^{3}\)
▶️Answer/Explanation
Ans:D
Question
0.25 g of anhydrous magnesium nitrate is heated strongly until it completely decomposes.
What is the total volume of gas produced, measured under room conditions?
A \(40cm^{3}\) B \(81cm^{3}\) C \(101^{3}\) D \(202^{3}\)
▶️Answer/Explanation
Ans:C
Question:
1.8 g of water, heated to 227 °C in a sealed container, turns to steam with a pressure of 200 kPa.
What is the approximate volume of the container?
A \(9~x~10^{-4}m^{3}\) B \(2~x~10^{3}m^{3}\) C \(2m^{3}\) D \(8~x~10^{7}m^{3}\)
▶️Answer/Explanation
Ans:C
Question:
Sodium azide, \(NaN_{3}\), decomposes as shown.
\(2NaN_{3}\rightarrow 2Na+3N_{2}\)
Which volume of nitrogen, measured at room temperature and pressure, will be produced by the decomposition of 150 g of sodium azide?
A \(166dm^{3}\) B \(83dm^{3}\) C \(55dm^{3}\) D \(37dm^{3}\)
▶️Answer/Explanation
Ans:B
Question
When a sample of a gas is compressed at constant temperature from $1500 \mathrm{kPa}$ to $6000 \mathrm{kPa}$, its volume changes from $76.0 \mathrm{~cm}^3$ to $20.5 \mathrm{~cm}^3$.
Which statements are possible explanations for this result?
1 The gas does not behave ideally.
2 The gas partially liquefies.
3 Some of the gas is lost from the container.
▶️Answer/Explanation
Ans:D
Question
Which gas sample contains the fewest molecules?
A $\quad 1.00 \mathrm{dm}^3$ of carbon dioxide at $27^{\circ} \mathrm{C}$ and $2.0 \mathrm{kPa}$
B $\quad 1.00 \mathrm{dm}^3$ of hydrogen at $100^{\circ} \mathrm{C}$ and $2.0 \mathrm{kPa}$
C $\quad 1.00 \mathrm{dm}^3$ of nitrogen at $300^{\circ} \mathrm{C}$ and $4.0 \mathrm{kPa}$
D $1.00 \mathrm{dm}^3$ of oxygen at $250^{\circ} \mathrm{C}$ and $3.0 \mathrm{kPa}$
▶️Answer/Explanation
Ans:B
Question
In the reaction shown, the concentrations of both X and Y are reduced to half of their original values whilst keeping the total volume of the solution constant.
\(X(aq) + Y(aq) \rightarrow XY(aq)\)
Simultaneously the temperature is increased from 298K to 348K.
Which prediction is definitely true?
A A smaller proportion of collisions between particles of X and particles of Y will be successful.
B The average kinetic energy of particles of X and particles of Y will increase.
C The rate of the reaction will be unaffected.
D The frequency of collisions between particles of X and particles of Y will halve.
Answer/Explanation
Ans: B
Question
The Boltzmann distribution for one mole of a gas at temperature T is shown.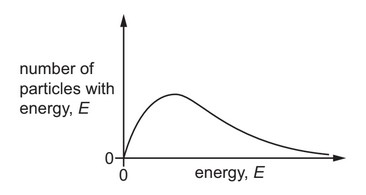
One mole of the same gas is added, and the gas remains at temperature T.
Which dotted curve shows the distribution with the added gas?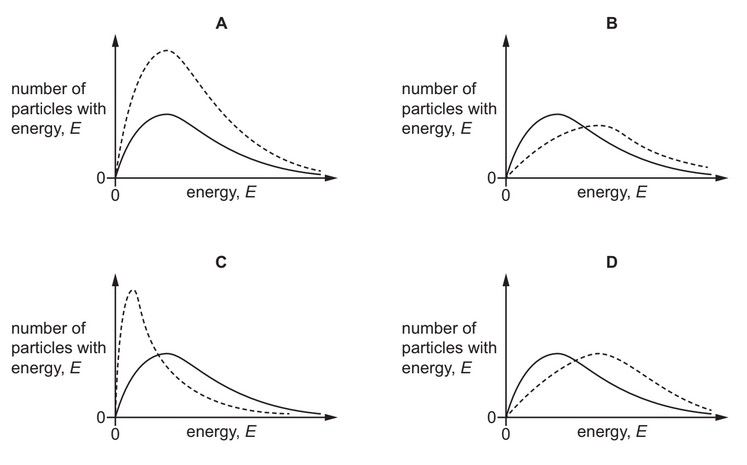
Answer/Explanation
Ans: A
Question
In the reaction shown, the concentrations of both X and Y are reduced to half of their original values whilst keeping the total volume of the solution constant.
\(X(aq) + Y(aq) \rightarrow XY(aq)\)
Simultaneously the temperature is increased from 298K to 348K.
Which prediction is definitely true?
A A smaller proportion of collisions between particles of X and particles of Y will be successful.
B The average kinetic energy of particles of X and particles of Y will increase.
C The rate of the reaction will be unaffected.
D The frequency of collisions between particles of X and particles of Y will halve.
Answer/Explanation
Ans: B
Question
The Boltzmann distribution for one mole of a gas at temperature T is shown.
One mole of the same gas is added, and the gas remains at temperature T.
Which dotted curve shows the distribution with the added gas?
Answer/Explanation
Ans: A
Question
17.6 g of pentan-1-ol is completely combusted.
Which volume of gaseous products is formed when measured at s.t.p.?
A 22.4 \(dm^3\) B 24.0 \(dm^3\) C 49.3 \(dm^3\) D 52.8 \(dm^3\)
Answer/Explanation
Ans: A
Question
The Boltzmann distribution for a gas at a constant temperature of \(50^o\) C is shown.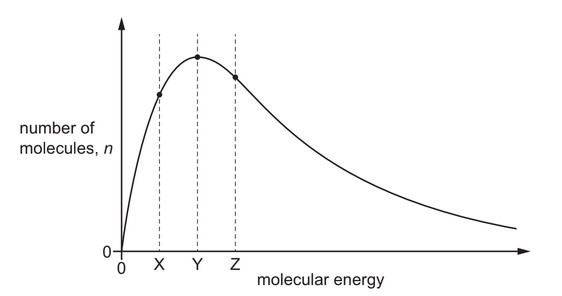
If the temperature of the gas is reduced by \(10^o\)C, the graph changes shape.
What happens to the values of n for the molecular energies X, Y and Z?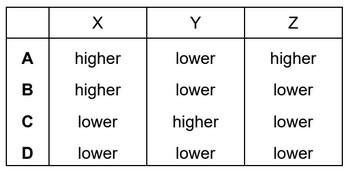
Answer/Explanation
Ans: B
Question
In separate experiments, 5.0 g samples of each of four s-block metals are added to an excess of water. The gas evolved is collected and its volume measured under the same conditions of temperature and pressure for each sample.
Which metal produces the largest volume of gas?
A calcium
B potassium
C rubidium
D strontium
Answer/Explanation
Ans: A
Question
Which gas would behave most like an ideal gas under room conditions?
A helium
B nitrogen
C ammonia
D krypton
Answer/Explanation
Answer A
Question
The diagram shows the Boltzmann distribution for the same gas at two different temperatures, T1 and T2.
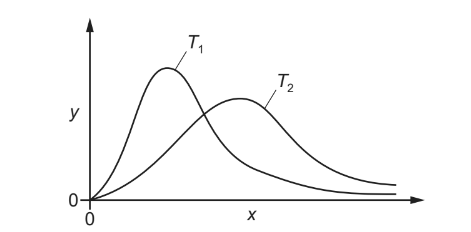
What is plotted on the y-axis and which line represents the higher temperature?
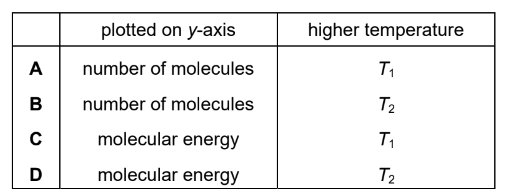
▶️Answer/Explanation
Answer: B
Question
Which substance shows the greatest deviation from the properties of an ideal gas under room conditions?
A CO2(g) B H2(g) C Ne(g) D NH3(g)
Answer/Explanation
Answer D
Question
The Boltzmann distribution is shown for a sample of gas at an initial temperature, T1.
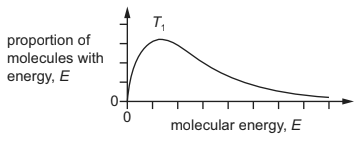
The sample of gas was heated to temperature, T2. What is the correct distribution for the higher temperature, T2?
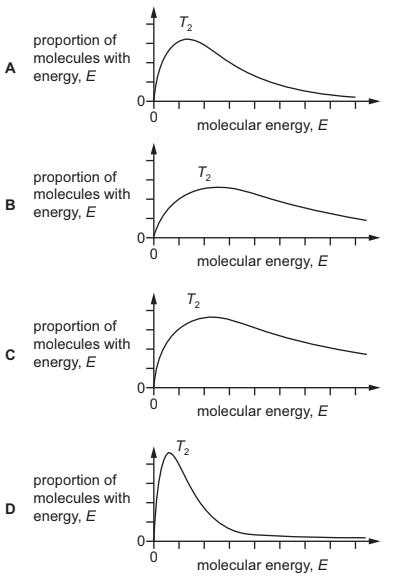
Answer/Explanation
Answer:
B
Question
Under which conditions will nitrogen behave most like an ideal gas?
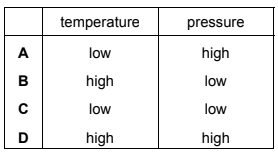
Answer/Explanation
Answer B
Question
When an evacuated tube of volume 400 cm3 is filled with gas at 300K and 101 kPa, the mass of the tube increases by 0.65 g.
Assume the gas behaves as an ideal gas.
What could be the identity of the gas?
A argon
B helium
C krypton
D neon
Answer/Explanation
Answer A
Question
The temperature of a sample of an inert gas is increased.
What effect does this have on the number of molecules with the most probable energy and on the number of molecules with high energy?
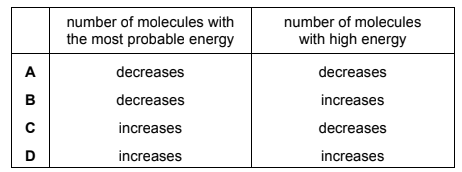
Answer/Explanation
Answer B
Question
In this question you should assume that all gases behave ideally.
Hydrogen and iodine react reversibly in the following reaction. The system reaches dynamic equilibrium.
\(H_{2}(g) + I_{2}(g) \rightleftharpoons 2HI(g)\) ∆H = –9.5 kJ mol–1
Which statement must be true for the Kp of this equilibrium to be constant?
A The partial pressures of H2, I2 and HI are equal.
B The external pressure is constant.
C The forward and reverse reactions have stopped.
D The temperature is constant.
Answer/Explanation
Answer D
Question
A container is partially filled with hot water, sealed and left to cool.
Which statements are correct?
1 As the temperature decreases, water molecules lose kinetic energy.
2 As the temperature decreases, more water molecules move from vapour to liquid.
3 As the temperature decreases, the vapour pressure of the water decreases.
The responses A to D should be selected on the basis of
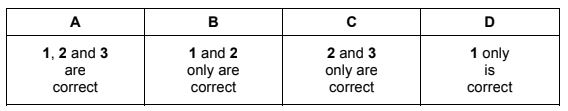
Answer/Explanation
Answer:
A
Question
The gas laws can be summarised in the ideal gas equation.
pV = nRT
0.960 g of oxygen gas is contained in a vessel of volume 7.00 × 10–3 m3 at a temperature of 30 °C.
Assume that the gas behaves as an ideal gas.
What is the pressure in the vessel?
A 1.07 kPa B 2.14 kPa C 10.8 kPa D 21.6 kPa
Answer/Explanation
Answer:
C
Question
What is a basic assumption of the kinetic theory, as applied to an ideal gas?
A Collisions between gas molecules are elastic.
B Each gas molecule occupies a finite volume.
C Gases consist of particles that experience the force of gravity.
D Gas molecules attract each other with weak intermolecular forces.
Answer/Explanation
Answer A
Question
The diagram shows the Boltzmann distribution of energies in a gas. The gas can take part in a reaction with an activation energy, Ea. The gas is maintained at a constant temperature.
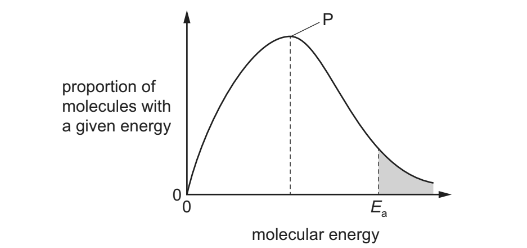
Which statement is correct?
A If a catalyst is added, peak P will be lower and Eawill move to the left.
B If a catalyst is added, peak P will be lower and Eawill move to the right.
C If a catalyst is added, peak P will be the same and Ea will move to the left.
D If a catalyst is added, peak P will be the same and Ea will move to the right.
Answer/Explanation
Answer C
Question
The diagram shows the Boltzmann distribution of energies in a gas. The gas can take part in a reaction with an activation energy, Ea. The gas is maintained at a constant temperature.

Which statement is correct?
A If a catalyst is added, peak P will be lower and Eawill move to the left.
B If a catalyst is added, peak P will be lower and Eawill move to the right.
C If a catalyst is added, peak P will be the same and Ea will move to the left.
D If a catalyst is added, peak P will be the same and Ea will move to the right.
Answer/Explanation
Answer C
Question
A fluorescent light tube has an internal volume of 400 \(cm^3\) and an internal pressure of 200 kPa. It is filled with 0.03 moles of an ideal gas.
What is the temperature of the gas inside the fluorescent light tube?
A 3.21 × \(10^{–1}\)K
B 3.21 × \(10^2\)K
C 3.21 × \(10^5\)K
D 3.21 × \(10^8\)K
Answer/Explanation
Ans: B
Question
In the ideal gas equation, pV = nRT, what are the units of n and T?
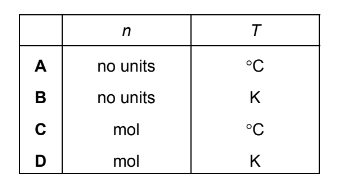
Answer/Explanation
Answer: D
Question
The equation shows a gas phase reaction.
X(g) → 2Y(g)
The diagram shows the Boltzmann distribution of a fixed mass of X(g) at temperature T in the absence of a catalyst. The line EA indicates the activation energy.
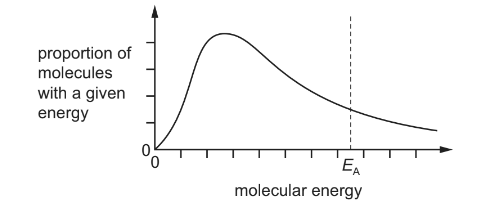
Which diagrams correctly show the effect of the following changes made separately and
independently?
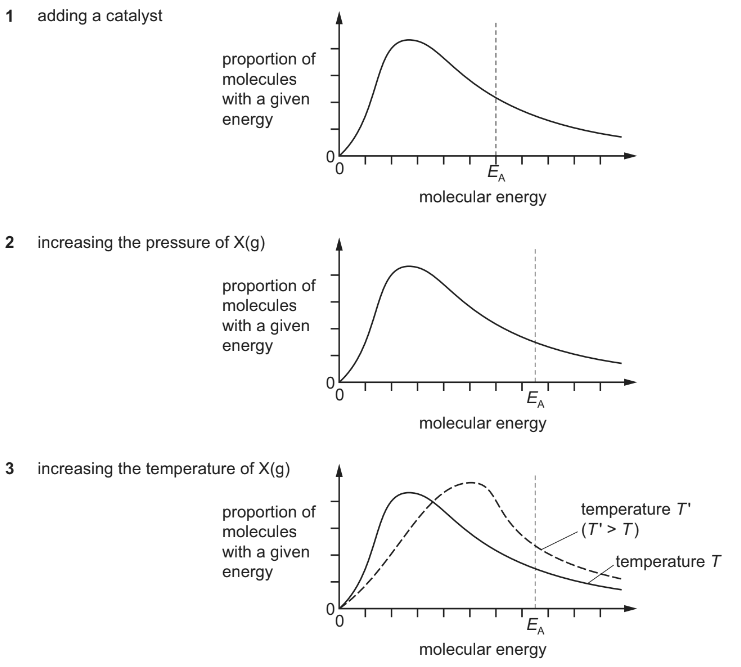
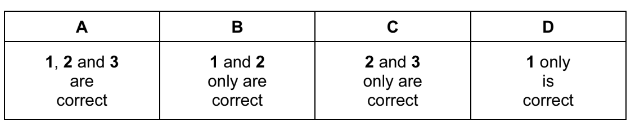
Answer/Explanation
Answer: B
Question
What are basic assumptions of the kinetic theory as applied to an ideal gas?
1 Gas particles are in continuous random motion.
2 Gas particles experience no intermolecular forces.
3 The volume of each gas particle is zero.

Answer/Explanation
Answer: A
Question
Use of the Data Booklet is relevant to this question. The gas laws can be summarised in the ideal gas equation below.
pV = nRT
0.96 g of oxygen gas is contained in a glass vessel of volume \( 7.0 × 10^{–3}m^{3}\) at a temperature of 30°C. Assume the gas behaves as an ideal gas.
What is the pressure in the vessel?
A 1.1kPa B 2.1kPa C 10.8kPa D 21.6kPa
Answer/Explanation
Ans:C
Question
The diagram shows a Boltzmann distribution of molecular energies for a gaseous mixture. The distribution has a peak, labelled P on the diagram.
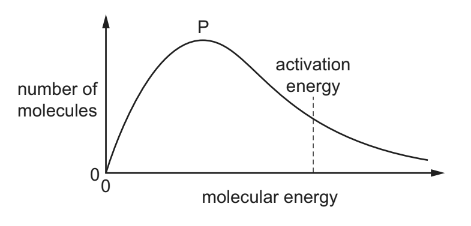
What happens when an effective catalyst is added to the mixture?
A The height of the peak decreases and the activation energy moves to the right.
B The height of the peak decreases and the activation energy moves to the left.
C The height of the peak remains the same and the activation energy moves to the right.
D The height of the peak remains the same and the activation energy moves to the left.
Answer/Explanation
Ans:D
Question
A student borrowed a friend’s chemistry notes and copied out the notes in the box below. Which statements are correct?
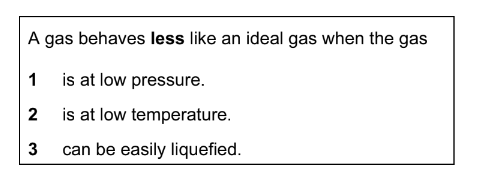
The responses A to D should be selected on the basis of
▶️Answer/Explanation
Ans:C
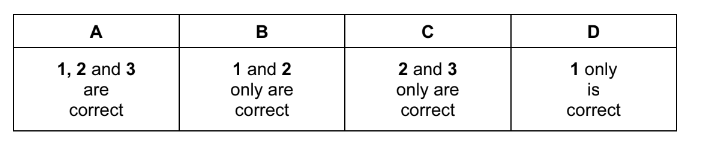
Question
Two glass vessels M and N are connected by a closed valve.
M contains helium at 20°C at a pressure of \91 × 10^{5}\)Pa. N has been evacuated, and has three
times the volume of M. In an experiment, the valve is opened and the temperature of the whole
apparatus is raised to 100°C.
What is the final pressure in the system?
A 3.18 × \(10^{4}\)Pa
B 4.24 ×\( 10^{4}\)Pa
C 1.25 × \(10^{5}\)Pa
D 5.09 ×\( 10^{5}\)Pa
Answer/Explanation
Ans:A
Question
Which solid-line curve most accurately represents the distribution of molecular energies in a gas at 500K if the dotted-line curve represents the corresponding distribution for the same gas at 300K?
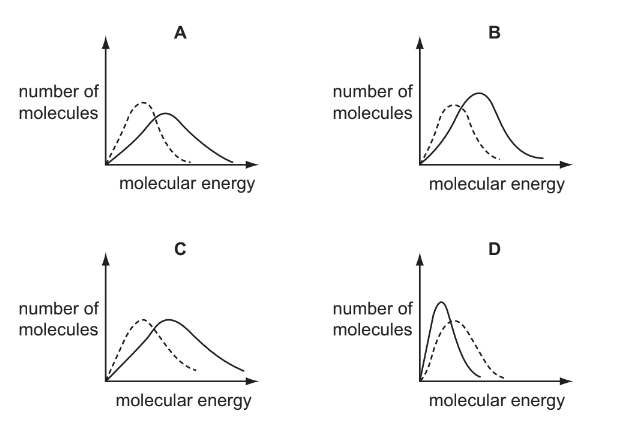
Answer/Explanation
Ans:A
Question
Boltzmann distributions are shown in the diagrams.
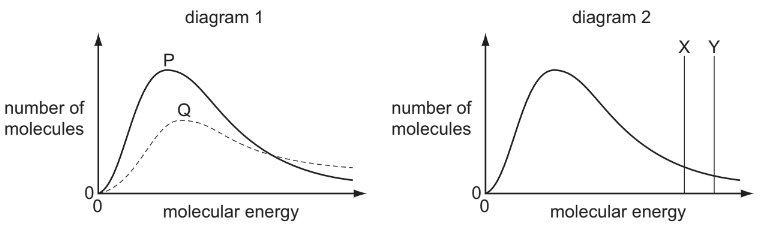
In diagram 1, one curve, P or Q, corresponds to a temperature higher than that of the other curve. In diagram 2, one line, X or Y, corresponds to the activation energy in the presence of a catalyst and the other line corresponds to the activation energy of the same reaction in the absence of a catalyst.
Which combination gives the correct curve and line?
Answer/Explanation
Ans:C
Question
The general gas equation can be used to calculate the Mr value of a gas.
For a sample of a gas of mass mg, which expression will give the value of \(M_r\)?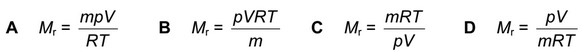
Answer/Explanation
Ans: B
Question
Which would behave the least like an ideal gas at room temperature?
A carbon dioxide
B helium
C hydrogen
D nitrogen
Answer/Explanation
Ans: A
Question
The diagrams below show the Boltzmann distribution for air at two temperatures.
The solid line represents the distribution at –20°C.
The dotted line represents the distribution at –10°C.
Which diagram is correct?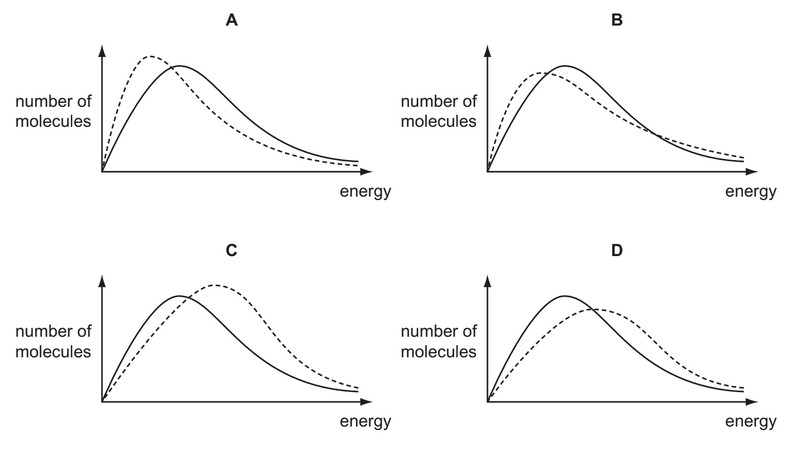
Answer/Explanation
Ans: D
Question
Use of the Data Booklet is relevant to this question.
1.15 g of a metallic element reacts with 300 cm3 of oxygen at 298 K and 1 atm pressure, to form an oxide which contains O2– ions.
What could be the identity of the metal?
- calcium
- magnesium
- potassium
- sodium
Answer/Explanation
Ans:
D
Question
Use of the Data Booklet is relevant to this question.
The volume of a sample of ammonia is measured at a temperature of 60°C and a pressure of 103kPa. The volume measured is 5.37 × 10–3m3.
What is the mass of the sample of ammonia, given to two significant figures?
A 0.00019g B 0.0034g C 0.19g D 3.4g
Answer/Explanation
Ans:
D
Question .
The gas laws can be summarised in the ideal gas equation.
pV = nRT
where each symbol has its usual meaning.
Which statements are correct?
- One mole of an ideal gas occupies the same volume under the same conditions of temperature and pressure.
- The density of an ideal gas at constant pressure is inversely proportional to the temperature, T.
- The volume of a given mass of an ideal gas is doubled if its temperature is raised from 25 °C to 50°C at constant pressure.
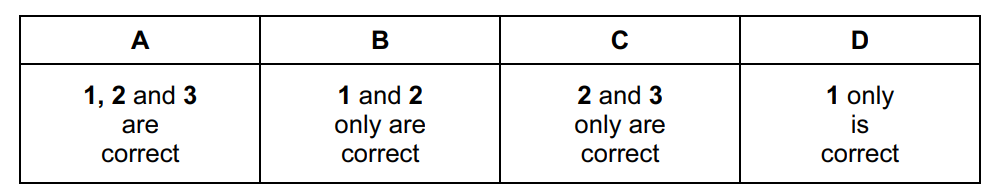
Answer/Explanation
Ans:
B
Question
Under which set of conditions is a gas most likely to behave ideally?
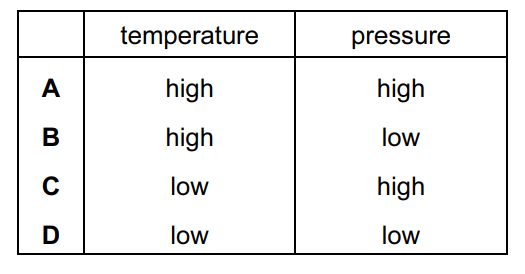
Answer/Explanation
Ans:
B
Question
Use of the Data Booklet is relevant to this question.
The gas laws can be summarised in the ideal gas equation.
pV = nRT
0.56g of ethene gas is contained in a vessel at a pressure of 102kPa and a temperature of 30°C.
What is the volume of the vessel?
A 49 cm3 B 494 cm3 C 48 900 cm3 D 494 000 cm3
Answer/Explanation
Ans:
B