Question
The rate of chemical reactions is affected by changes in temperature and pressure.
(a) (i) Draw a curve on the axes to show the Boltzmann distribution of energy of particles in a sample of gaseous krypton atoms at a given temperature.
Label the curve T1 and label the axes.

(ii) On the diagram in (a)(i), draw a second curve to show the distribution of energies of the krypton atoms at a higher temperature.
Label the second curve T2.
(b) The Boltzmann distribution assumes that the particles behave as an ideal gas.
(i) State two assumptions of the kinetic theory as applied to an ideal gas.
(ii) 2.00g of krypton gas, Kr(g), is placed in a sealed 5.00dm³ container at 120°C.
Calculate the pressure, in Pa, of Kr(g) in the container.Assume Kr(g) behaves as an ideal gas.
Show your working.
(iii) State and explain the conditions at which krypton behaves most like an ideal gas.
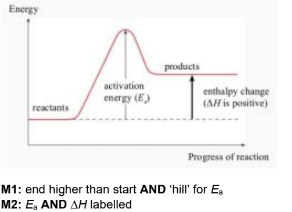
(c) Krypton reacts with fluorine in the presence of ultraviolet light to make krypton difluoride, KrF2(g).
\(Kr(g) + F_{2} (g) \rightarrow KrF_{2}(g)\)
activation energy for the reaction, Ea = +385kJmol-1
enthalpy change of formation of KrF2, ∆Hf = +60.2kJmol-1
(i) Use this information to complete the reaction profile diagram for the formation of KrF2. Label Ea and ∆Hf on the diagram.
Assume the reaction proceeds in one step.
(ii) Explain, in terms of activation energy, Ea, and the collision of particles, how an increase in temperature affects the rate of a chemical reaction.
Answer/Explanation
Answer: (a)(i)
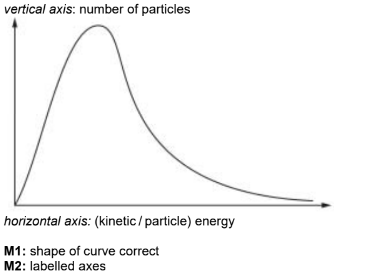
(a)(ii) Labelled line (T2) with lower peak to right of original
(b)(i) Any two from: • no VdW forces present / no forces of attraction between particles
• (ideal gas) particles have no / negligible volume (compared to container)
• collisions between (ideal gas) particles / walls of container are perfectly elastic
• (ideal gas) particles behave as rigid spheres
(b)(ii)

(b)(iii) M1: low pressure AND high temperature
M2: Either of:
• volume of particles is negligible (compared to volume of container)
• VdW forces are insignificant (owing to high kinetic energy of particles)
(c)(i)
(c)(ii) • rate increases • (increase in temperature means) more particles have energy ⩾ activation energy • frequency of successful collisions increases
Question
Carbon monoxide gas, CO(g), and nitrogen gas, N2(g), are both diatomic molecules.
(a) The diagram shows the arrangement of outer electrons in a molecule of CO(g).
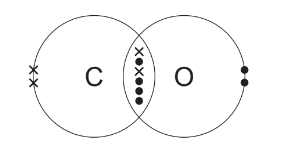
(i) State one similarity and one difference in the way the atoms in a carbon monoxide molecule are bonded together compared to the atoms in a nitrogen molecule.
(ii) The table states the electronegativity values of carbon, nitrogen and oxygen atoms.
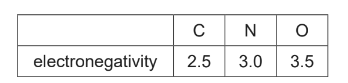
Use the electronegativity values and relevant details from the Data Booklet to complete the table below.
(b) N2(g) is less reactive than CO(g) even though N2(g) has a lower bond energy than CO(g). Suggest why CO(g) is more reactive than N2(g).
(c) Both carbon monoxide and nitrogen are gases at room temperature and pressure.
They both behave like ideal gases under certain conditions.
(i) State the two conditions necessary for these two gases to approach ideal gas behaviour.
(ii) Explain why N2(g) behaves more like an ideal gas than CO(g) does at 20.0°C and 101kPa.
(d) Calculate the amount, in mol, of pure nitrogen gas which occupies 100cm³ at 101kPa and 20.0°C. Use relevant information from the Data Booklet. Show your working.
Assume nitrogen behaves as an ideal gas.
Answer/Explanation
Answer: (a)(i) M1 both make triple (covalent) bond / 3 shared pairs of electrons 1
M2 one bond in CO is coordinate / dative covalent / formed by donating a pair of electrons from O (to C)
(a)(ii)
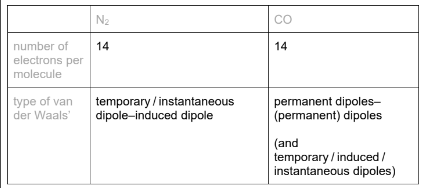
(b) CO / it is a polar molecule / it has a (permanent) dipole (but N2 is non-polar)
(c)(i) high temperature AND low pressure
(c)(ii) M1 CO is polar / has a permanent dipole OR N2 is non-pola
M2 IMF in CO are (more) significant / larger OR IMF in N2 are smaller / less significant
Alternative answer
M1 (Size of) N2 smaller than CO
OR volume of N2 molecules / particles smaller
Alternative answer
M2 volume of N2 molecules / particles is more negligible
ORA
(d) M1 correct conversion to consistent units
P = 101 000 V = 100 / 1 000 000 = (1 × 10-4) T = 293
M2 use of all values from M1 in correct relationship, n = PV / RT
M3 calculation = 4.15 × 10-3 mol