Question
Solid carbon dioxide, $\mathrm{CO}_2$, is similar to solid iodine, $\mathrm{I}_2$, in its structure and properties. Carbon is in Group 14. Silica, $\mathrm{SiO}_2$, is a Group 14 compound.
Which statement about solid $\mathrm{CO}_2$ and solid $\mathrm{SiO}_2$ is correct?
A Both solids exist in a lattice structure.
B Both solids have a simple molecular structure.
C Both solids have atoms joined by single covalent bonds.
D Both solids change spontaneously to gas at s.t.p.
▶️Answer/Explanation
Ans:A
Question
P, Q and R represent three different structures of an element.
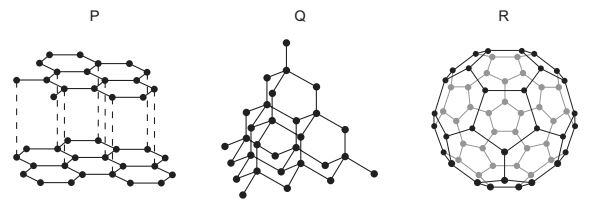
Which structures are giant molecular?
A P, Q and R
B P and Q only
C P and R only
D Q and R only
Answer/Explanation
Answer:
B
Question
Which row describes the structure and bonding of SiO2 and SiCl4?
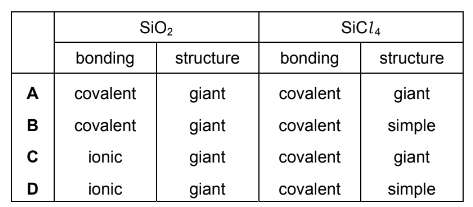
Answer/Explanation
Answer B
Question
Silicon is heated in an excess of chlorine, producing compound J.
Excess water is added to the sample of J produced.
Which row is correct?
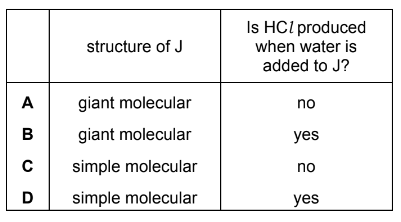
Answer/Explanation
Answer D
Question
Graphene, graphite and the fullerene \(C_{60}\) are allotropes of carbon.
Which statements are correct for all three of these allotropes of carbon?
1 Delocalised electrons are present in the structure.
2 All bond angles are 120°.
3 It has a giant molecular crystalline lattice structure.
The responses A to D should be selected on the basis of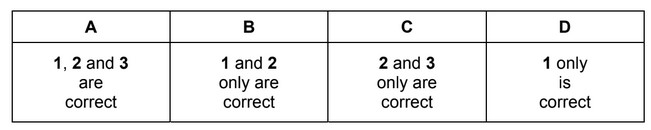
No other combination of statements is used as a correct response.
Answer/Explanation
Ans: D
Question
Which solid contains more than one type of bonding?
A iodine
B silicon dioxide
C sodium chloride
D zinc
Answer/Explanation
Answer A
Question
In which structure are three atoms bonded together in a straight line?
A poly(ethene),\((-CH_2CH_2-)_n\)
B propane, \(C_3H_8\)
C silicon tetrachloride, \(SiCl_4\)
D sulfur hexafluoride, \(SF_6\)
Answer/Explanation
Ans: D
Question
Dicarbon monoxide, C2O, is found in dust clouds in space. The structure of this molecule is C=C=O. The molecule contains no unpaired electrons.
How many lone pairs of electrons are present in a molecule of C2O?
A 1 B 2 C 3 D 4
Answer/Explanation
Answer: C
Question
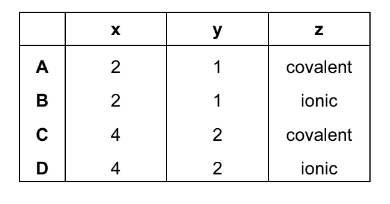
Some car paints contain small flakes of silica, \(SiO_{2}\).
In the structure of solid \(SiO_{2}\)
● each silicon atom is bonded to x oxygen atoms,
● each oxygen atom is bonded to y silicon atoms,
● each bond is a z type bond.
What is the correct combination of x, y and z in these statements?
Answer/Explanation
Ans:C
Question
Use of the Data Booklet is relevant to this question.
The bond energy of the Br–O bond is 235kJ \(mol^{–1}\).
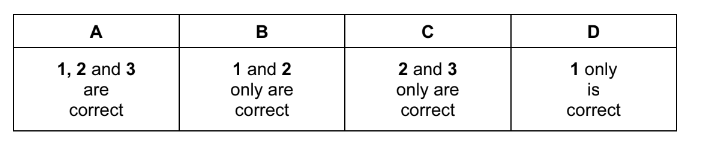
Which reactions are exothermic?
1 OH• + HBr → H2 + BrO•
2 OH• + HBr → H2O + Br•
3 H• + HBr → H2 + Br•
The responses A to D should be selected on the basis of
▶️Answer/Explanation
Ans:C
Question
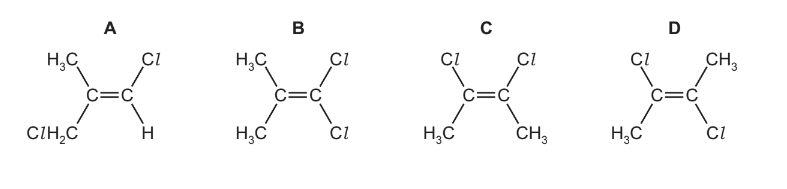
Which molecular structure will have the smallest overall dipole?
Answer/Explanation
Ans:D
Question
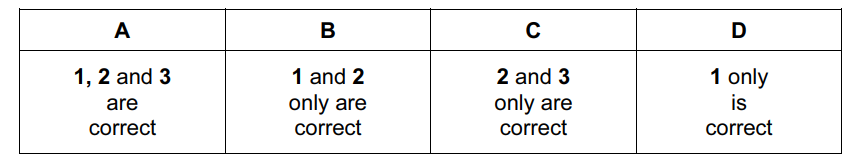
Which of these substances have a giant structure?
- silicon(IV) oxide
- baked clay found in crockery
- phosphorus(V) oxide
Answer/Explanation
Ans:
B
Question
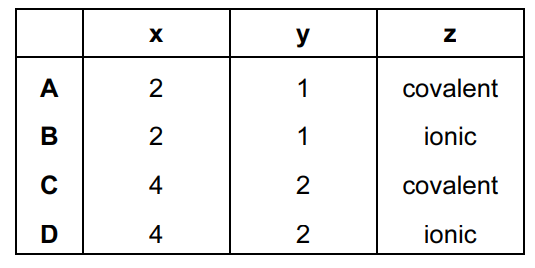
Some car paints contain small flakes of silica, SiO2.
In the structure of solid SiO2
- each silicon atom is bonded to x oxygen atoms,
- each oxygen atom is bonded to y silicon atoms,
- each bond is a z type bond.
What is the correct combination of x, y and z in this statement?
Answer/Explanation
Ans:
C