Question:
An experiment was performed to determine the enthalpy of combustion of ethanol.
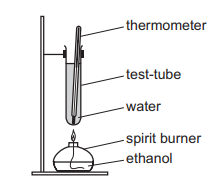
The data collected are shown.
mass of water $=W$ g
mass of ethanol burned $=X \mathrm{~g}$
temperature rise $=Y^{\circ} \mathrm{C}$
molar mass of ethanol $=\mathrm{Zgmol}^{-1}$
specific heat capacity of water $=4.2 \mathrm{JK}^{-1} \mathrm{~g}^{-1}$
Which expression can be used to calculate the enthalpy of combustion of ethanol in $\mathrm{kJmol}^{-1}$ ?
A $\frac{-4.2 W Y Z}{1000 X}$
B $\frac{-4.2 W Y X}{1000 Z}$
C $\frac{-4.2 X Y Z}{1000 W}$
D $\frac{-4.2 X(Y+273) Z}{1000 W}$
▶️Answer/Explanation
Ans:A
Question
Nitrogen forms a number of oxides. Their enthalpies of formation are given.
$
\begin{aligned}
& \Delta H_{\mathrm{f}}^\theta[\mathrm{NO}(\mathrm{g})]=+90 \mathrm{~kJ} \mathrm{~mol}^{-1} \\
& \Delta H_{\mathrm{f}}^{\mathrm{\theta}}\left[\mathrm{N}_2 \mathrm{O}(\mathrm{g})\right]=+82 \mathrm{~kJ} \mathrm{~mol}^{-1} \\
& \Delta H_{\mathrm{f}}^{\mathrm{i}}\left[\mathrm{NO}_2(\mathrm{~g})\right]=+33 \mathrm{~kJ} \mathrm{~mol}^{-1}
\end{aligned}
$
Which statements are correct?
1 If $\mathrm{N}_2 \mathrm{O}(\mathrm{g})$ is oxidised by $\mathrm{O}_2(\mathrm{~g})$ to $\mathrm{NO}_2(\mathrm{~g}), 16 \mathrm{~kJ}$ is released per mole of $\mathrm{N}_2 \mathrm{O}$.
2 The decomposition of $\mathrm{N}_2 \mathrm{O}(\mathrm{g})$ to $\mathrm{N}_2(\mathrm{~g})$ and $\mathrm{O}_2(\mathrm{~g})$ is exothermic.
3 The reaction between $\mathrm{NO}$ and oxygen is exothermic.
▶️Answer/Explanation
Ans:A
Question
Which statements are correct?
1 enthalpy of combustion of $\mathrm{H}_2=$ enthalpy of formation of $\mathrm{H}_2 \mathrm{O}$
2 enthalpy of formation of $\mathrm{H}_2=-$ (enthalpy of atomisation of $\mathrm{H}_2$ )
3 enthalpy of solution of $\mathrm{HCl}=$ enthalpy of hydration of $\mathrm{H}^{+}+$enthalpy of hydration of $\mathrm{Cl}^{-}$

▶️Answer/Explanation
Ans:D
Question:
An energy cycle for the combustion of methane is shown.
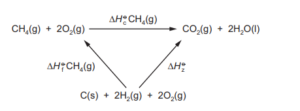
Which expressions can be used to calculate the energy change, \(\Delta H_{z}^{\Theta }\)
1 \(\Delta H_{f}^{\Theta }CH_{4}(g)+\Delta H_{c}^{\Theta }CH_{4}(g)\)
2 \(\Delta H_{c}^{\Theta }C(s)+2\Delta _{c}^{\Theta }H_{2}(g)\)
3 \(\Delta H_{c}CO(g)~+~1\Delta H_{c}^{\Theta }H_{2}(g)\)
▶️Answer/Explanation
Ans:B
Question
Which pair of standard enthalpy changes are numerically equal?
A atomisation of $\mathrm{CH}_4(\mathrm{~g})$ and formation of $\mathrm{CH}_4(\mathrm{~g})$
B combustion of $\mathrm{CH}_3 \mathrm{OH}(\mathrm{l})$ and combustion of graphite + 2(combustion of $\mathrm{H}_2(\mathrm{~g})$ )
C combustion of graphite and formation of $\mathrm{CO}_2(\mathrm{~g})$
D neutralisation of $\mathrm{HCl}(\mathrm{aq})$ with $\mathrm{NaOH}(\mathrm{aq})$ and formation of $\mathrm{H}_2 \mathrm{O}(\mathrm{l})$
▶️Answer/Explanation
Ans:C
Question
An energy cycle is drawn for the following reaction.
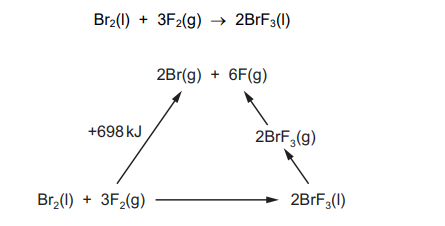
The standard enthalpy of formation of $\mathrm{BrF}_3(\mathrm{l})=-301 \mathrm{~kJ} \mathrm{~mol}^{-1}$.
The enthalpy change of $\mathrm{BrF}_3(\mathrm{l})$ to $\mathrm{BrF}_3(\mathrm{~g})$ is $+44 \mathrm{~kJ} \mathrm{~mol}^{-1}$.
What is the average bond energy of the $\mathrm{Br}-\mathrm{F}$ bond in $\mathrm{BrF}_3$ ?
A $152 \mathrm{~kJ} \mathrm{~mol}^{-1}$
B $202 \mathrm{~kJ} \mathrm{~mol}^{-1}$
C $304 \mathrm{~kJ} \mathrm{~mol}^{-1}$
D $404 \mathrm{~kJ} \mathrm{~mol}^{-1}$
▶️Answer/Explanation
Ans:B
Question
Carbon monoxide burns readily in oxygen to form carbon dioxide.
What does this information suggest?
1 The +4 oxidation state of carbon is more stable than the +2 state.
2 The standard enthalpy change of formation of carbon dioxide is more negative than the
standard enthalpy change of formation of carbon monoxide.
3 The value of the equilibrium constant for the reaction, \(2CO(g)~+~O_{2}(g)\rightleftharpoons 2CO_{2}(g)\), is likely to be high.
▶️Answer/Explanation
Ans:A
Question
Two reactions are shown.
\(H_{2}(g)\rightarrow 2H(g)\)
\(CO(g)~+~\frac{1}{2}O_{2}(g)\rightarrow CO_{2}(g)\)
If molar amounts are used, how can the two energy changes associated with these reactions be
described?
A enthalpy of atomisation and enthalpy of combustion
B enthalpy of atomisation and enthalpy of formation
C bond energy and enthalpy of combustion
D bond energy and enthalpy of formation
▶️Answer/Explanation
Ans:C
Question:
A reaction between carbon and oxygen is shown.
\(C(s)~+~\frac{1}{2}O_{2}g\rightarrow CO(g)\)
How can the standard enthalpy change of this reaction be described correctly?
1 standard enthalpy change of formation
2 standard enthalpy change of combustion
3 standard enthalpy change of atomisation

▶️Answer/Explanation
Ans:D
Question:
The following data are needed for this question.
\(NaHCO_{3}(s)~+~ HC \imath(aq)\rightarrow NaC \imath(aq)~+~H_{2}O(l)~+~CO_{2}g ~~~\Delta H = 38.97 kJ~mol^{-1}\)
On heating, sodium hydrogencarbonate decomposes as shown
\(NaHCO_{3}(s)\rightarrow Na_{2}CO_{3}(s)~+~H_{2}O(l)+CO_{2}(g)\)
What is the enthalpy change for this decomposition?
A \(-57.62kJ~mol^{-1}\)
B \(-18.65kJ~mol^{-1}\)
C \(18.65kJ~mol^{-1}\)
D \(57.62kJ~mol^{-1}\)
▶️Answer/Explanation
Ans:C
Question
All the reactants and products of an exothermic reaction are gaseous.
Which statement about this reaction is correct?
A The total bond energy of the products is less than the total bond energy of the reactants, and $\Delta H$ for the reaction is negative.
B The total bond energy of the products is less than the total bond energy of the reactants, and $\Delta H$ for the reaction is positive.
C The total bond energy of the products is more than the total bond energy of the reactants, and $\Delta H$ for the reaction is negative.
D The total bond energy of the products is more than the total bond energy of the reactants, and $\Delta H$ for the reaction is positive.
▶️Answer/Explanation
Ans:C
Question
A student mixed $25.0 \mathrm{~cm}^3$ of $4.00 \mathrm{moldm}^{-3}$ hydrochloric acid with an equal volume of $4.00 \mathrm{~mol} \mathrm{dm}^{-3}$ sodium hydroxide. The initial temperature of both solutions was $15.0^{\circ} \mathrm{C}$. The maximum temperature recorded was $30.0^{\circ} \mathrm{C}$.
Using these results, what is the enthalpy change of neutralisation of hydrochloric acid?
A $-62.7 \mathrm{~kJ} \mathrm{~mol}^{-1}$
B $-31.4 \mathrm{~kJ} \mathrm{~mol}^{-1}$
C $-15.7 \mathrm{~kJ} \mathrm{~mol}^{-1}$
D $-3.14 \mathrm{~kJ} \mathrm{~mol}^{-1}$
▶️Answer/Explanation
Ans:B
Question
The diagram illustrates the enthalpy changes of a set of reactions
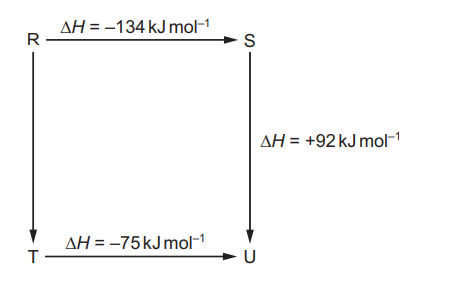
Which statements are correct?
1 The enthalpy change for the transformation $U \rightarrow R$ is $+42 \mathrm{~kJ} \mathrm{~mol}^{-1}$.
2 The enthalpy change for the transformation $T \rightarrow S$ is endothermic.
3 The enthalpy change for the transformation $\mathrm{R} \rightarrow \mathrm{T}$ is $-33 \mathrm{~kJ} \mathrm{~mol}^{-1}$.
The responses A to D should be selected on the basis of
No other combination of statements is used as a correct response.
▶️Answer/Explanation
Ans:D
Question
Which equation has an enthalpy change of reaction which corresponds to the standard enthalpy change of atomisation of chlorine?
A $\frac{1}{2} \mathrm{Cl}_2(\mathrm{~g}) \rightarrow \mathrm{Cl}(\mathrm{g})$
B $ \frac{1}{2} \mathrm{Cl}_2(\mathrm{l}) \rightarrow \mathrm{Cl}(\mathrm{g})$
C $\mathrm{Cl}_2(\mathrm{~g}) \rightarrow 2 \mathrm{Cl}(\mathrm{g})$
D $\mathrm{Cl}_2(\mathrm{l}) \rightarrow 2 \mathrm{Cl}(\mathrm{g})$
▶️Answer/Explanation
Ans:A
Question
The equation for reaction 1 is shown.
reaction 1 \(C_6H_{12}O_6 \rightarrow 2CO_2 + 2C_2H_5OH\)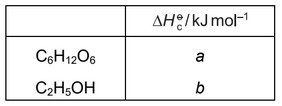
What is the correct expression for the enthalpy change of reaction 1?
A a + b B a – b C a + 2b D a – 2b
Answer/Explanation
Ans: D
Question
The equation for an enthalpy change is shown. The enthalpy change is Q.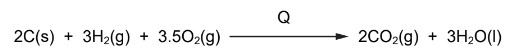
What is the correct expression to calculate Q?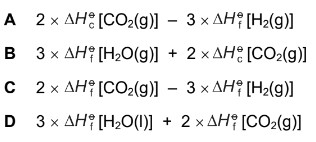
Answer/Explanation
Ans: D
Question
\(\Delta H_{1}^{\theta }\) is the standard enthalpy of formation of methane.
\(\Delta H_{2}^{\theta }\) is the standard enthalpy of combustion of carbon.
\(\Delta H_{3}^{\theta }\) is the standard enthalpy of combustion of hydrogen.
\(CH_{4}(g) + 2O_{2}(g) \xrightarrow[]{\Delta H_{C}^{\theta }} CO_{2}(g) + 2H_{2}O(I)\)
Which expression is equivalent to ?
A \(\Delta H_{1}^{\theta } – \Delta H_{2}^{\theta } + \Delta H_{3}^{\theta }\)
B \(\Delta H_{1}^{\theta } – 2\Delta H_{3}^{\theta } + \Delta H_{2}^{\theta }\)
C \(\Delta H_{2}^{\theta } – \Delta H_{3}^{\theta } + \Delta H_{1}^{\theta }\)
D \(\Delta H_{2}^{\theta } + 2\Delta H_{3}^{\theta } – \Delta H_{1}^{\theta }\)
Answer/Explanation
Answer: D
Question
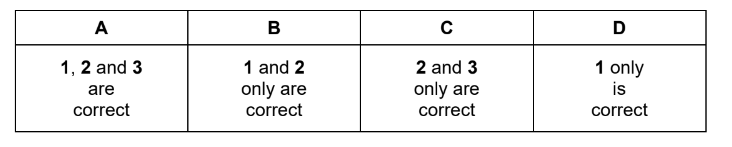
The diagram shows an incomplete energy profile diagram for a reaction.
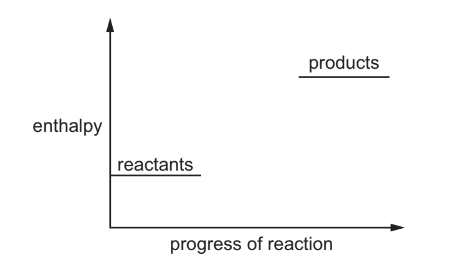
Which reactions could this diagram refer to?
1 \(CaCO_{3}(s) \rightarrow CaO(s) + CO_{2}(g)\)
2 \(H_{2}(g) \rightarrow 2H(g)\)
3 \(Cl^{-}(aq) \rightarrow Cl^{-}(g) + aq\)
Answer/Explanation
Answer: A
Question
Which equation represents the enthalpy change of atomisation of iodine?
A \(\frac{1}{2}I_{2}(g) \rightarrow I(g)\)
B \(I_{2}(g)\rightarrow 2I(g)\)
C \(\frac{1}{2}I_{2}(s) \rightarrow I(g)\)
D \(I_{2}(s)\rightarrow 2I(g)\)
Answer/Explanation
Answer C
Question
The equation for a chemical reaction is shown. All substances are in their standard states.
XeF6 + 3H2O → XeO3 + 6HF
Which statement describes the standard enthalpy change of reaction for this reaction?
A the enthalpy change when a total of one mole of products is produced
B the enthalpy change when a total of one mole of reactants is reacted
C the enthalpy change when one mole of water reacts
D the enthalpy change when six moles of hydrogen fluoride are produced
Answer/Explanation
Answer:
D
Question
The following data are needed for this question.
(N2H4(l)) = 50.6 kJ mol–1
(N2O4(g)) = 9.2 kJ mol–1
(H2O(g)) = –241.8 kJ mol–1
Hydrazine, N2H4(l), reacts with dinitrogen tetraoxide, N2O4(g), to form nitrogen gas and water vapour.
2N2H4(l) + N2O4(g) → 3N2(g) + 4H2O(g)
What is the enthalpy change for this reaction?
A –1077.6 kJ mol–1
B –856.8 kJ mol–1
C –301.6 kJ mol–1
D –182.0 kJ mol–1
Answer/Explanation
Answer A
Question
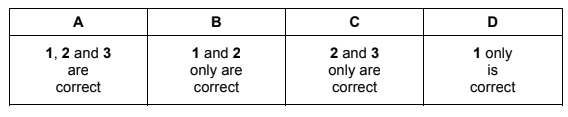
Which statements about enthalpy changes are correct?
1 The enthalpy change of atomisation is always positive.
2 The enthalpy change when a C–C bond is broken is positive.
3 The enthalpy change of neutralisation of a weak acid is always negative.
Answer/Explanation
Answer A
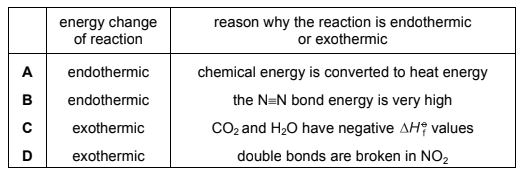
Question
At 550 °C nitrogen dioxide reacts with unburnt hydrocarbon fragments such as CH3 in the catalytic converter of a motor vehicle.
4CH3 + 7NO2 → 3\(\frac{1}{2}\)N2 + 4CO2 + 6H2O
Which row gives the energy change for this reaction and a possible reason for it?
Answer/Explanation
Answer C
Question
Two reactions and their enthalpy changes are shown.
2C(s) + 2H2(g) → C2H4(g) ∆HΘ= +52.2 kJ mol–1
C2H2(g) + H2(g) → C2H4(g) ∆HΘ= –175.8 kJ mol–1
These data can be used to calculate the enthalpy change for the reaction shown.
2C(s) + H2(g) → C2H2(g) ∆HΘ= X
What is the value of X?
A –228.0 kJ mol–1
B –123.6 kJ mol–1
C +123.6 kJ mol–1
D +228.0 kJ mol–1
Answer/Explanation
Answer D
Question
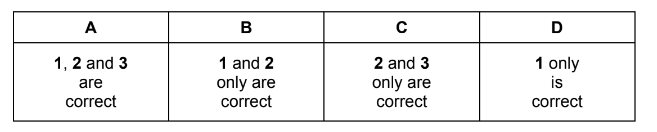
Which statements are correct for all exothermic reactions?
1 ∆H for the reaction is negative.
2 On a reaction pathway diagram the products are shown lower than the reactants.
3 The reaction will occur without heating.
Answer/Explanation
Answer B
Question
Calcium reacts with water to form calcium hydroxide and hydrogen.
Ca(s) + 2H2O(l) → Ca(OH)2(s) + H2(g)
The standard enthalpy change for this reaction is – 414kJ mol–1.
What further information is needed in order to calculate the standard enthalpy change of formation of calcium hydroxide, \(\Delta H_{t}^{\Theta }\)Ca(OH)2(s)?
1 \(\Delta H_{t}^{\Theta }\) for H2O(l)
2 \(\Delta H_{t}^{\Theta }\)for H2(g)
3 first and second ionisation energies of Ca
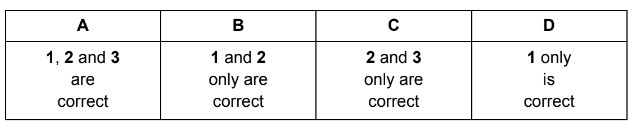
Answer/Explanation
Answer D
Question
Enthalpy changes of combustion can be used to determine enthalpy changes of formation. The following equation represents the enthalpy change of formation of butane.
4C(s) + 5H2(g) → C4H10(g)
By using the following standard enthalpy of combustion data, what is the value of the standard enthalpy change of formation, \(\Delta H_{t}^{\Theta }\) , of butane?
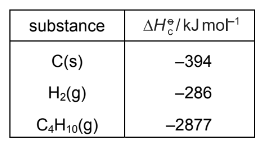
A –5883 kJ mol–1
B –129 kJ mol–1
C +129 kJ mol–1
D +2197 kJ mol–1
Answer/Explanation
Answer B
Question
Which equation represents the standard enthalpy change of formation of water?
A H2(g) + \(\frac{1}{2}\)O2(g) → H2O(g)
B H2(g) + \(\frac{1}{2}\)O2(g) → H2O(l)
C 2H2(g) + O2(g) → 2H2O(g)
D 2H2(g) + O2(g) → 2H2O(l)
Answer/Explanation
Answer:
B
Question
Sulfur can be oxidised in two ways.
\(S(s) + O_2(g) → SO_2(g)\) \(∆H^o\) = –296.5 kJ \(mol^{–1}\)
\(2S(s) + 3O_2(g) → 2SO_3(g)\) \(∆H^o\) = –791.4 kJ \(mol^{–1}\)
Sulfur trioxide can be made from sulfur dioxide and oxygen.
\(2SO_2(g) + O_2(g) → 2SO_3(g)\)
What is the standard enthalpy change for this reaction?
A –1384.4 kJ \(mol^{–1}\)
B –989.8 kJ \(mol^{–1}\)
C –494.9 kJ \(mol^{–1}\)
D –198.4 kJ \(mol^{–1}\)
Answer/Explanation
Ans: D
Question
A reaction between carbon and oxygen is shown.
\(C(s) + \frac{1}{2} O_2(g) → CO(g)\)
How can the enthalpy change of this reaction be described correctly?
1 enthalpy change of formation
2 enthalpy change of combustion
3 enthalpy change of atomisation
The responses A to D should be selected on the basis of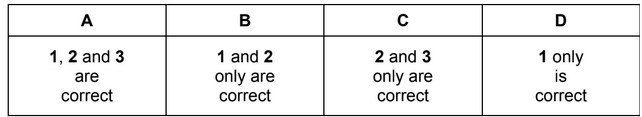
Answer/Explanation
Ans: D
Question
Which expression gives the standard enthalpy change of combustion of methane?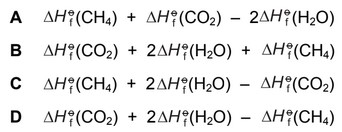
Answer/Explanation
Ans: D
Question
For which enthalpy changes is the value of ∆H always negative?
1 combustion
2 hydration
3 solution
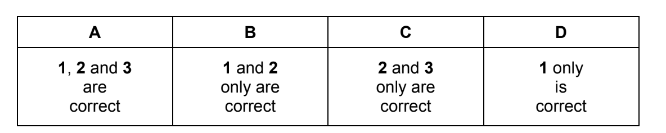
Answer/Explanation
Answer: B
Question
At 550 °C nitrogen dioxide reacts with unburnt hydrocarbon fragments such as CH$_3$• in the catalytic converter of a motor vehicle.
\(4CH_{3}\cdot + 7NO_{2} \rightarrow 3\frac{1}{2}N_{2} + 4CO_{2} + 6H_{2}O\)
The following table lists types of energy change for this reaction and possible reasons for them.
Which row gives the energy change for this reaction and the reason for it?
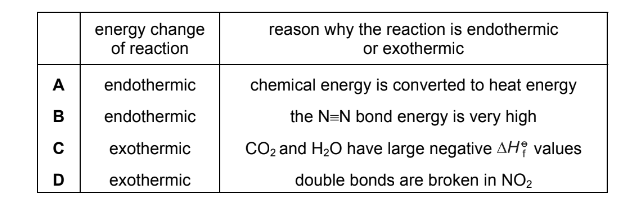
Answer/Explanation
Answer: C
Question
Which equation shows the reaction that occurs during the standard enthalpy change of atomisation of bromine?
A Br2(l) → 2Br(g)
B Br2(g) → 2Br(g)
C \(\frac{1}{2}\)Br2(l) → Br(g)
D \(\frac{1}{2}\)Br2(g) → Br(g)
Answer/Explanation
Answer: C
Question
Which quantity gives the best indication of the relative strengths of the hydrogen bonds between the molecules in liquid hydrogen halides?
A bond dissociation energies
B enthalpy changes of formation
C enthalpy changes of solution
D enthalpy changes of vaporisation
Answer/Explanation
Ans:D
Question
Use of the Data Booklet is relevant for this question.
In an experiment, the burning of 1.45g (0.025mol) of propanone was used to heat 100g of water. The initial temperature of the water was 20.0°C and the final temperature of the water was 78.0°C.
Which experimental value for the enthalpy change of combustion for propanone can be calculated from these results?
A \( –1304kJ mol^{–1}\)
B \(–970kJ mol^{–1}\)
C \(–352kJ mol^{–1}\)
D \(–24.2kJ mol^{–1}\)
Answer/Explanation
AnsB
Question
For which equation is the enthalpy change correctly described as an enthalpy change of formation?
A \(9C(g) + O_{2}(g) → CO_{2}\)(g)
B C(s) +\ Frac{1}{2} O2(g) → CO(g)
C \( 2N(g) + 4O(g) → N_{2}O_{4}(g)\)
D \(2NO(g) + O_{2}(g) → 2NO_{2}\)(g)
Answer/Explanation
Ans:B
Question
In an experiment to calculate the enthalpy change of combustion of a fuel, 1.5g (0.0326mol) of the fuel was used to heat 200g of water. The temperature of the water rose from 25°C to 55°C. The specific heat capacity of water is \(4.18Jg^{–1}K^{–1}\). There is significant heat loss in this experiment. Therefore, the experimental value for the enthalpy change of combustion, ∆Hc, of the fuel will be different from the theoretical value.
Using the information above, what is the \experimental value for the enthalpy change of combustion, ∆Hc, of the fuel?
A –1410kJ\( mol^{–1}\)
B –769kJ\( mol^{–1}\)
C –30.7kJ \(mol^{–1}\)
D –16.7kJ\( mol^{–1}\)
Answer/Explanation
Ans:B
Question
Ethanol is increasingly being used as a fuel for cars.
The standard enthalpy change of formation of carbon dioxide is – \(393kJmol^{–1}\).
The standard enthalpy change of formation of water is –\(286kJ mol^{–1}\).
The standard enthalpy change of formation of ethanol is \(–277kJ mol^{–1}\).
What is the standard enthalpy change of combustion of ethanol?
A –1921kJ \(mol^{–1}\)
B –1367kJ \(mol^{–1}\)
C –956kJ\( mol^{–1}\)
D –402kJ\( mol^{–1}\)
Answer/Explanation
Ans:B
Question
A student mixed \(25.0cm^{3}\) of 0.350 \(moldm^{–3}\) sodium hydroxide solution with \(25.0cm^{3}\) of 0.350 \(moldm^{–3}\) hydrochloric acid. The temperature rose by 2.50°C. Assume that no heat was lost
to the surroundings. The final mixture had a specific heat capacity of 4.20J \(cm^{3}\)\(K^{–1}\).
What is the molar enthalpy change for the reaction?
A –150kJ \(mol^{–1}\)
B –60.0kJ\( mol^{–1}\)
C –30.0kJ\( mol^{–1}\)
D –0.150kJ\( mol^{–1}\)
Answer/Explanation
Ans:B
Question
Enthalpy changes that are difficult to measure directly can often be determined using Hess’ Law to construct an enthalpy cycle. Which enthalpy change is indicated by X in the enthalpy cycle shown?
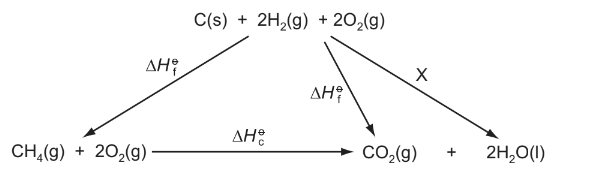
A –4 × the enthalpy of combustion of hydrogen
B +4 × the enthalpy of combustion of hydrogen
C – 2 × the enthalpy of formation of water
D +2 × the enthalpy of formation of water
Answer/Explanation
Ans:D
Question
Use of the Data Booklet is relevant to this question.
This question should be answered using bond enthalpy data. The equation for the complete combustion of methane is given below.
\(CH_4 + 2O_2 → CO_2 + 2H_2O\)
What is the enthalpy change of combustion of methane?
A –1530kJ \(mol^{–1}\)
B –1184kJ \(mol^{–1}\)
C –770kJ \(mol^{–1}\)
D –688kJ \(mol^{–1}\)
Answer/Explanation
Ans: D
Question .
Which process could be used to calculate the bond energy for the covalent bond X-Y by dividing its ∆H by n?
- XYn(g) → X(g) + nY(g)
- 2XYn(g) → 2XYn-1(g) + Y2(g)
- Y(g) + XYn-1(g) → XYn(g)
- nXY(g) → nX(g) + \(\frac{n}{2}\) Y2(g)
Answer/Explanation
Ans:
A
Question
A student calculated the standard enthalpy change of formation of ethane, C2H6, using a method based on standard enthalpy changes of combustion.
He used correct values for the standard enthalpy change of combustion of ethane (–1560 kJ mol–1) and hydrogen (–286 kJ mol–1) but he used an incorrect value for the standard enthalpy change of combustion of carbon. He then performed his calculation correctly. His final answer was –158kJ mol–1.
What did he use for the standard enthalpy change of combustion of carbon?
- –1432kJ mol–1
- –860kJ mol–1
- –430kJ mol–1
- –272kJ mol–1
Answer/Explanation
Ans:
C
Question
In the table below,
- ‘+’ means that this type of standard enthalpy change can only have positive values,
- ‘–’ means that this type of standard enthalpy change can only have negative values,
- ‘+/ –’ means that either positive or negative values are possible.
Which row is correct?
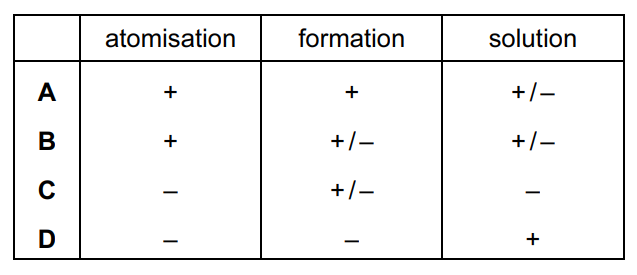
Answer/Explanation
Ans:
B
Question
The equation for a reaction is shown.
\(H_{2}\left ( g \right )+\frac{1}{2}O_{2}\left ( g \right )\rightarrow H_{2}O\left ( I \right );\Delta H=xkJmol^{-1}\)
Which pair of descriptions is fully correct for this reaction?
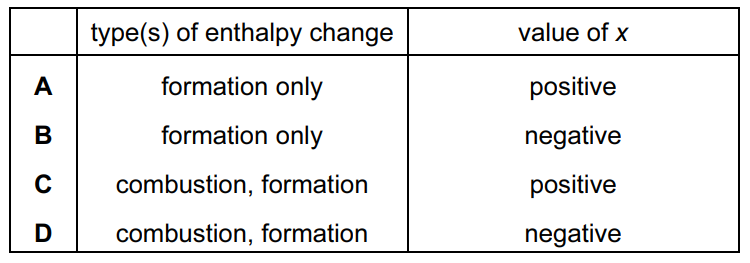
Answer/Explanation
Ans:
D
Question
Propanone has the molecular formula C3H6O.
The enthalpy change of combustion of hydrogen is –286kJmol–1.
The enthalpy change of combustion of carbon is –394kJ mol–1.
The enthalpy change of combustion of propanone is –1786kJ mol–1.
Using this information, what is the enthalpy change of formation of propanone?
- –1106kJ mol–1
- –540kJ mol–1
- –254kJ mol–1
- +1106kJ mol–1
Answer/Explanation
Ans:
C