Question
Hydrogen halides are compounds formed when halogens (Group 17 elements) react with hydrogen.
The bond polarity of the hydrogen halides decreases from HF to HI.
Some relevant data are shown in the table
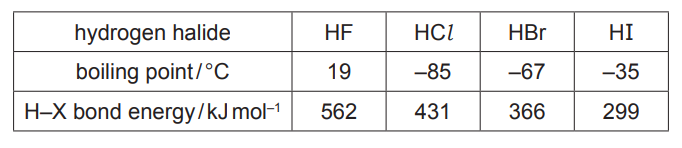
(a) (i) Explain the meaning of the term bond polarity [1]
(ii) Suggest why the boiling point of HF is much higher than the boiling points of the other hydrogen halides. [2]
(iii) Describe and explain the relative thermal stabilities of the hydrogen halides.
(b) The equation for the preparation of hydrogen chloride using concentrated sulfuric acid is shown.
$
\mathrm{H}_2 \mathrm{SO}_4+\mathrm{NaCl} \rightarrow \mathrm{NaHSO}_4+\mathrm{HCl}
$
(i) Use the Brønsted-Lowry theory of acids and bases to identify the base and its conjugate acid in this reaction. Explain your answer.
Brønsted-Lowry base $($ base-I) $=$
conjugate acid $($ acid-II) $=$[2]
(ii) Explain why the reaction of concentrated sulfuric acid and sodium iodide is not suitable for the preparation of hydrogen iodide.[2]
(c) Hydrogen chloride undergoes a reversible reaction with oxygen
$
4 \mathrm{HCl}(\mathrm{g})+\mathrm{O}_2(\mathrm{~g}) \rightleftharpoons 2 \mathrm{Cl}_2(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{g})
$
The reaction is carried out at $400^{\circ} \mathrm{C}$ in the presence of a copper(II) chloride catalyst.
(i) Use the data in the table to calculate the overall enthalpy change of reaction.
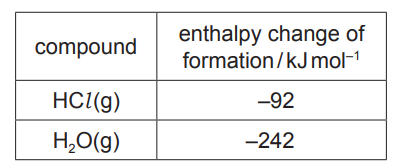
enthalpy change of reaction $=$
$\mathrm{kJmol}^{-1}[2]$
(ii) State the type of catalyst used in this reaction. Explain how a catalyst is able to increase the rate of a chemical reaction.[2]
(iii) The reaction exists in dynamic equilibrium.
The reaction was repeated at $1000^{\circ} \mathrm{C}$ and the same pressure.
State and explain the effect on the composition of the equilibrium mixture of the change in temperature. [2]
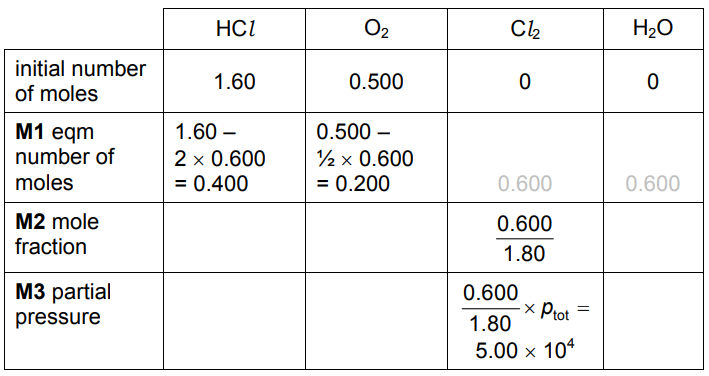
(iv) When $1.60 \mathrm{~mol}$ of $\mathrm{HCl}$ are mixed in a sealed container with $0.500 \mathrm{~mol}$ of $\mathrm{O}_2$ at $400^{\circ} \mathrm{C}$, $0.600 \mathrm{~mol}$ of $\mathrm{Cl}_2$ and $0.600 \mathrm{~mol}$ of $\mathrm{H}_2 \mathrm{O}$ are formed.
The total pressure inside the container is $1.50 \times 10^5 \mathrm{~Pa}$.
- Calculate the amounts, in mol, of $\mathrm{HCl}$ and $\mathrm{O}_2$ in the equilibrium mixture.
$
\begin{aligned}
& \mathrm{HCl}=\ldots \ldots \ldots \ldots \ldots \ldots \ldots \ldots . \ldots \mathrm{mol} \\
& \mathrm{O}_2=\ldots \ldots \ldots \ldots \ldots \ldots \ldots \ldots \ldots \mathrm{mol}
\end{aligned}
$
$\mathrm{mol}$
- Calculate the mole fraction of $\mathrm{Cl}_2$ and hence the partial pressure of $\mathrm{Cl}_2$ in the equilibrium mixture.
(v) In a separate experiment, an equilibrium reaction mixture was found to contain the four gases at the partial pressures shown in the table.
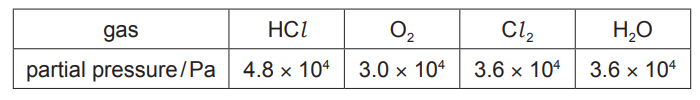
$
K_{\mathrm{p}}=\frac{\left(p_{\mathrm{Cl}}\right)^2 \times\left(p_{\mathrm{H}_2 \mathrm{O}}\right)^2}{\left(p_{\mathrm{HCl}}\right)^4 \times \mathrm{p}_{\mathrm{O}_2}}
$
Use this information and the expression given for $K_p$ to calculate a value for $K_p$. State the units of $K_{\mathrm{p}}$.
$
K_{\mathrm{p}}=
$
units $=$[2]
(vi) The reaction is repeated without a catalyst.
State the effect of this on $K_p$.[1] [Total: 22]
▶️Answer/Explanation
Ans:
(a)(i) bond in which the centres of positive and negative charges do not coincide
OR electron distribution is asymmetric /unequal
OR two (bonded) atoms are partially charged
(a)(ii) HF has the strongest (permanent) dipole–dipole/ van der Waals’
(forces)/HF has hydrogen bonding requires more energy to overcome (than weaker (permanent) dipole–dipole/van der Waals’ forces between other hydrogen halides)
(a)(iii) thermal stability of the hydrogen halides decreases down group (17) 1
larger (halogen) atoms /atomic radius (down group) / increased shielding 1
bond energies decrease / less energy required to break H–X
(b)(i)
M1 base is $\mathrm{Cl}^{-}$AND conjugate acid is $\mathrm{HCl}$ OR base is $\mathrm{HSO}_4^{-}$AND conjugate acid is $\mathrm{H}_2 \mathrm{SO}_4$
M2 $\mathrm{Cl}^{-} / \mathrm{HSO}_4^{-} /$base is a proton acceptor OR $\mathrm{HC} l / \mathrm{H}_2 \mathrm{SO}_4 /$ (conjugate) acid has one more $\mathrm{H}^{+}$
(b)(ii)
$\mathrm{H}_2 \mathrm{SO}_4$ is (too strong) an oxidising agent
$I_2$ would be formed instead
(c)(i) $\quad \Delta_{\mathrm{r}} \mathrm{H}=\Delta_{\mathrm{r}} \mathrm{H}$ \{products $\}-\Delta_{\mathrm{r}} \mathrm{H}\{$ reactants $\}=2 \times(-242)-4 \times(-92)$
= –116 (sign AND answer)
(c)(ii) heterogeneous (catalyst) 1
provides an alternative reaction pathway of lower activation energy 1
(c)(iii) reaction is exothermic
(increased temperature) shifts equilibrium to the left AND decreases yield of products $\left(\mathrm{Cl}_2\right.$ and /or $\left.\mathrm{H}_2 \mathrm{O}\right) /$ less product formed
(c)(iv)
(c)(v) $K_p=\frac{\left(3.6 \times 10^4\right)^2 \times\left(3.6 \times 10^4\right)^2}{\left(4.8 \times 10^4\right)^4 \times 3.0 \times 10^4}=1.05 \times 10^{-5}$
units $=\mathrm{Pa}^{-1}$
2(c)(vi) $\quad K_p$ would not change
Question
Phosphorus, sulfur and chlorine can all react with oxygen to form oxides.
(a) Phosphorus reacts with an excess of oxygen to form phosphorus(V) oxide.
(i) Write an equation to show the reaction of phosphorus with excess oxygen.[1]
(ii) Describe the reaction of phosphorus(V) oxide with water.[2]
(iii) State the structure and bonding of solid phosphorus(V) oxide.[1]
(b) The two most common oxides of sulfur are $\mathrm{SO}_2$ and $\mathrm{SO}_3$.
When $\mathrm{SO}_2$ dissolves in water, a small proportion of it reacts with water to form a weak Brønsted-Lowry acid.
(i) Explain the meaning of the term weak Brønsted-Lowry acid.[2]
(ii) Write the equation for the reaction of $\mathrm{SO}_2$ with water.$[1]$
(iii) $\mathrm{SO}_2$ reacts with $\mathrm{NO}_2$ in the atmosphere to form $\mathrm{SO}_3$ and $\mathrm{NO}$.
$\mathrm{NO}$ is then oxidised in air to form $\mathrm{NO}_2$.
$
\begin{gathered}
\mathrm{SO}_2+\mathrm{NO}_2 \rightarrow \mathrm{SO}_3+\mathrm{NO} \\
2 \mathrm{NO}+\mathrm{O}_2 \rightarrow 2 \mathrm{NO}_2
\end{gathered}
$
State the role of $\mathrm{NO}_2$ in this two-stage process. [1]
(c) Emissions of $\mathrm{SO}_2$ from coal-fired power stations can be reduced by mixing the coal with powdered limestone.
Limestone is heated to form $\mathrm{CaO}$ in reaction 1. This then reacts with $\mathrm{SO}_2$ and $\mathrm{O}_2$ to form $\mathrm{CaSO}_4$ in reaction 2.
reaction 1: $\quad \mathrm{CaCO}_3(\mathrm{~s}) \rightarrow \mathrm{CaO}(\mathrm{s})+\mathrm{CO}_2(\mathrm{~s})$
reaction 2: $\mathrm{CaO}(\mathrm{s})+\mathrm{SO}_2(\mathrm{~g})+\frac{1}{2} \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CaSO}_4(\mathrm{~s})$
(i) State the type of reaction occurring in reaction 1.[1]
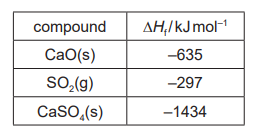
(ii) Use the data to calculate the enthalpy change of reaction 2 .
(d) Chlorine forms several oxides, including $\mathrm{Cl}_2 \mathrm{O}, \mathrm{ClO}_2$ and $\mathrm{Cl}_2 \mathrm{O}_6$.
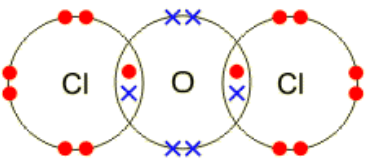
(i) Draw a ‘dot-and-cross’ diagram of $\mathrm{Cl}_2 \mathrm{O}$. Show outer-shell electrons only.[1]
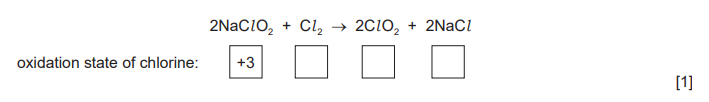
(ii) $\mathrm{ClO}_2$ can be prepared by reacting $\mathrm{NaClO}_2$ with $\mathrm{Cl}_2$.
Write the oxidation state of chlorine in each species in the boxes provided.
(iii) $\mathrm{Cl}_2 \mathrm{O}_6(\mathrm{~g})$ is produced by the reaction of $\mathrm{ClO}_2(\mathrm{~g})$ with $\mathrm{O}_3(\mathrm{~g})$.
$
2 \mathrm{ClO}_2(\mathrm{~g})+2 \mathrm{O}_3(\mathrm{~g}) \rightleftharpoons \mathrm{Cl}_2 \mathrm{O}_6(\mathrm{~g})+2 \mathrm{O}_2(\mathrm{~g}) \quad \Delta H=-216 \mathrm{~kJ} \mathrm{~mol}^{-1}
$
The reaction takes place at $500 \mathrm{~K}$ and $100 \mathrm{kPa}$.
State and explain the effect on the yield of $\mathrm{Cl}_2 \mathrm{O}_6(\mathrm{~g})$ when the experiment is carried out:
- at $1000 \mathrm{~K}$ and $100 \mathrm{kPa}$
- at $500 \mathrm{~K}$ and $500 \mathrm{kPa}$. [4]
(e) Element E is a Period 5 element.
E reacts with oxygen to form an insoluble white oxide that has a melting point of $1910^{\circ} \mathrm{C}$. The oxide of $\mathbf{E}$ conducts electricity only when liquid.
$\mathrm{E}$ also reacts readily with $\mathrm{Cl}_2(\mathrm{~g})$ to form a white solid that reacts exothermically with water. The resulting solution reacts with aqueous silver nitrate to form a white precipitate that dissolves in dilute ammonia.
(i) Suggest the type of bonding shown by the oxide of E. Explain your answer.[2]
(ii) Suggest the type of bonding shown by the chloride of E. Explain your answer.[2] [Total: 21]
▶️Answer/Explanation
Ans:
$(\mathrm{a})(\mathrm{i}) \quad \mathrm{P}_4+5 \mathrm{O}_2 \rightarrow \mathrm{P}_4 \mathrm{O}_{10}$
(a)(ii) any two from:
• reacts vigorously
• solid disappears / colourless solution forms
• hydrolysis
• exothermic
• acid(ic) (solution)
• steamy / misty fumes
(a)(iii) Simple and covalent OR molecular and covalent
(b)(i) \text { M1: proton / } \mathrm{H}^{+} \text {donor }
M2: partially dissociates (in solution)
(b)(ii) $\mathrm{SO}_2+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{H}_2 \mathrm{SO}_3$
(b)(iii) (homogeneous) catalyst 1
(c)(i) thermal decomposition
(c)(ii) M1: $\Delta H_r=-1434-(-635+-297)$
M2: $=-502\left(\mathrm{~kJ} \mathrm{~mol}^{-1}\right)$
(d)(i)
(d)(ii)
![]()
(d)(iii) (at 1000 K and 100 kPa)
M1: (yield) decreases
M2: reaction is exothermic AND equilibrium moves left
(at 500 K and 500 kPa)
M3: (yield) increases
M4: fewer moles (of gas) on right-hand side AND equilibrium moves right
(e)(i) M1: ionic
M2: ions only able / free to move / free to conduct (when liquid / molten)
(e)(ii) M1: covalent
M2: hydrolysed (by water)