Question
Ammonia is manufactured from nitrogen and hydrogen using the Haber process.
$
\mathrm{N}_2(\mathrm{~g})+3 \mathrm{H}_2(\mathrm{~g}) \rightleftharpoons 2 \mathrm{NH}_3(\mathrm{~g})
$
What is the expression for $K_{\mathrm{c}}$ for this equilibrium?
A $\frac{2\left[\mathrm{NH}_3(\mathrm{~g})\right]}{\left[\mathrm{N}_2(\mathrm{~g})\right]+3\left[\mathrm{H}_2(\mathrm{~g})\right]}$
B $\frac{2\left[\mathrm{NH}_3(\mathrm{~g})\right]}{\left[\mathrm{N}_2(\mathrm{~g})\right] \times 3\left[\mathrm{H}_2(\mathrm{~g})\right]}$
c $\frac{\left[\mathrm{NH}_3(\mathrm{~g})\right]^2}{\left[\mathrm{~N}_2(\mathrm{~g})\right]+\left[\mathrm{H}_2(\mathrm{~g})\right]^3}$
D $\frac{\left[\mathrm{NH}_3(\mathrm{~g})\right]^2}{\left[\mathrm{~N}_2(\mathrm{~g})\right] \times\left[\mathrm{H}_2(\mathrm{~g})\right]^3}$
▶️Answer/Explanation
Ans:D
Question
Nitrogen monoxide is an atmospheric pollutant that is formed inside car engines by an endothermic reaction between nitrogen and oxygen.
\(N_2(g) + O_2(g) \rightarrow 2NO(g)\)
Which diagram correctly represents the energy profile for this reaction?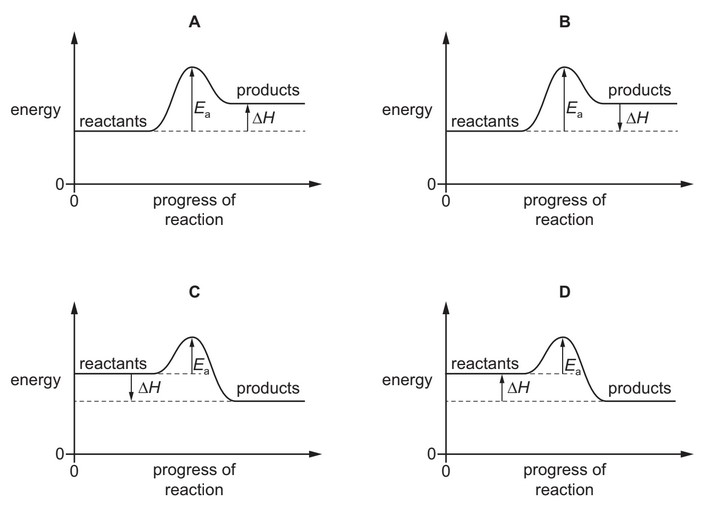
Answer/Explanation
Ans: A
Question
A reaction pathway diagram for the reaction of aqueous sodium hydroxide and dilute sulfuric acid is shown.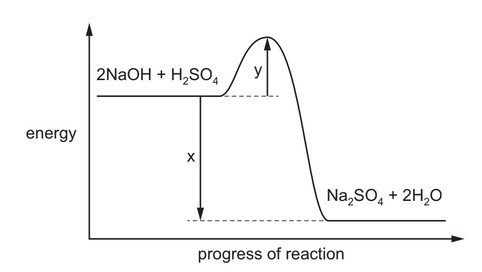
What is the value of the enthalpy change of neutralisation, H$_{\text{neut}}$?
A x B x – y C \(\frac{x}{2}\) D \(\frac{(x y)}{2}\)
Answer/Explanation
Ans: C
Question
The diagram represents the Haber process for the manufacture of ammonia from nitrogen and hydrogen.
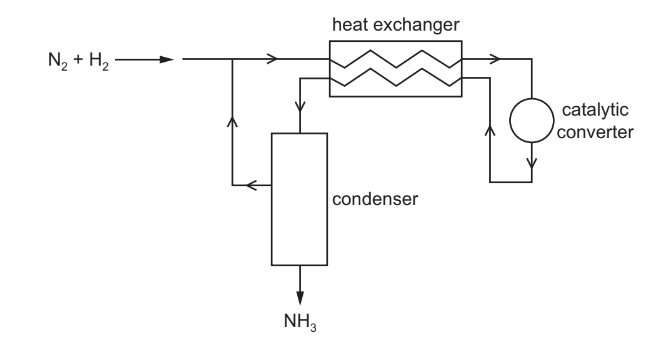
What is the purpose of the heat exchanger?
A to cool the incoming gas mixture to avoid overheating the catalyst
B to cool the reaction products and separate the NH3 from unused N2 and H2
C to warm the incoming gas mixture and shift the equilibrium to give more NH3
D to warm the incoming gas mixture and speed up the reaction
Answer/Explanation
Answer: D
Question
The standard enthalpy changes of combustion of carbon, hydrogen and methanol are shown.
C(s) + O2(g) → CO2(g) \(\Delta H_{\Theta }^{c}\) = –394 kJ mol–1
H2(g) + \(\frac{1}{2}\) O2(g) → H2O(l) \(\Delta H_{\Theta }^{c}\) = –286kJ mol–1
CH3OH(l) + 1\(\frac{1}{2}\) O2(g) → CO2(g) + 2H2O(l) \(\Delta H_{\Theta }^{c}\) = –726kJ mol–1
Which expression gives the standard enthalpy change of formation of methanol in kJ mol–1?
A –394 + (–286) – (–726)
B –394 + (–286 × 2) – 726
C –394 + (–286 × 2) – (–726)
D –726 – (–394) – (–286 × 2)
Answer/Explanation
Answer:
C
Question
Hess’ Law and bond energy data can be used to calculate the enthalpy change of a reaction.
Bromoethane, CH3CH2Br, can be made by reacting ethene with hydrogen bromide.
CH2=CH2 + HBr → CH3CH2Br
What is the enthalpy change for this reaction?
A – 674kJ mol–1
B – 64kJ mol–1
C +186 kJ mol–1
D +346 kJ mol–1
Answer/Explanation
Answer:
B
Question
200 g of water are at 25°C.
The water is heated to 75 °C by burning 2 g of ethanol.
What is the amount of energy transferred to the water?
A 0.418 kJ B 10.4 kJ C 41.8 kJ D 62.7 kJ
Answer/Explanation
Ans: C
Question
The table shows the partial pressures in an equilibrium mixture formed by the Haber process.
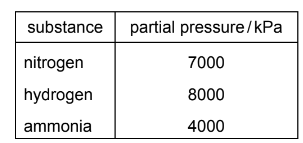 \(3H_{2}(g) + N_{2}(g) \rightleftharpoons 2NH_{3}(g)\)
\(3H_{2}(g) + N_{2}(g) \rightleftharpoons 2NH_{3}(g)\)
What is the numerical value of the equilibrium constant, Kp, for this reaction?
A 4.46 × 10–9
B 4.76 × 10–5
C 7.14 × 10–5
D 2.24 × 108
Answer/Explanation
Answer: A
Question
An important reaction in the manufacture of nitric acid is the catalytic oxidation of ammonia.
\(4NH_{3}(g) + 5O_{2}(g) \rightleftharpoons 4NO(g) + 6H_{2}O(g)\)
For every mole of O2 that reacts in this way, 181.8 kJ of energy are released.
A factory makes 2.50 × 105 mol of NO every day.
How much energy, in kJ, is released every day?
A 3.64 × 107 B 4.55 × 107 C 5.68 × 107 D 2.27 × 108
Answer/Explanation
Answer: C
Question
The standard enthalpy changes of combustion of glucose and ethanol are given as –2820 and \(–1368kJ mol^{–1}0 respectively.
Glucose, \(C_{6}H_{12}O_{6} ,\) can be converted into ethanol.
![]()
What is the standard enthalpy change for this reaction?
A –1452kJ \( mol^{–1}\)
B –84kJ \( mol^{–1}\)
C +84kJ \(mol^{–1}\)
D +1452kJ \(mol^{–1}\)
Answer/Explanation
Ans:B
Question
Solid sulfur consists of molecules made up of eight atoms covalently bonded together. The bonding in sulfur dioxide is O=S=O.
enthalpy change of combustion of S_{8}, \(\Delta H_{c}^{\Theta }S_{8}(s) \) = \(–2376kJmol^{-1}\)
energy required to break 1 mole \(S_{8}\)(s) into gaseous atoms = 2232kJ \(mol^{–1}\)
O=O bond enthalpy = \(496kJ mol^{–1}\)
Using these data, what is the value of the S=O bond enthalpy?
A \(239kJ mol^{–1}\) B \(257kJ mol^{–1}\) C \( 319kJ mol^{–1}\) D \(536kJ mol^{–1}\)
Answer/Explanation
Ans:D
Question
Which set of conditions gives the highest yield of ammonia at equilibrium?
\(N_{2}(g) + 3H_{2}(g) \rightleftharpoons 2NH_{3}(g) \) \( ∆H_o =–92kJ mol^{–1}\)
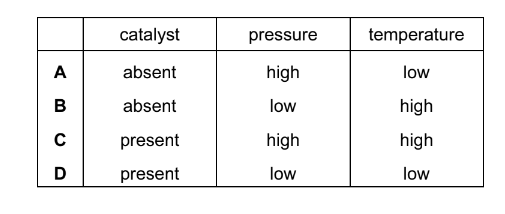
Answer/Explanation
Ans:A
Question
Use of the Data Booklet is relevant to this question.
A student carried out an experiment to determine the enthalpy change for the combustion of
methanol.
The following results were obtained by the student.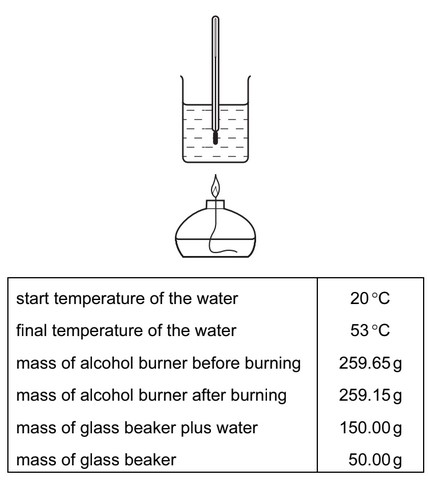
How much of the heat energy produced by the burning of methanol went into the water?
A 209J B 13 794J C 20 691J D 22 154J
Answer/Explanation
Ans: B
Question
Use of the Data Booklet is relevant to this question.
A reaction which causes the presence of oxides of nitrogen in car exhausts is the formation of NO.
N2 + O2 → 2NO ∆H = +180kJ mol–1
What is the bond energy in kJmol–1 of the bond between the atoms in NO?
A 655 B 835 C 1310 D 1670
Answer/Explanation
Ans:
A