Question
Nitric acid, \(HNO_{3}\) can be made by reacting nitrogen dioxide with water.
The enthalpy change for the reaction can be measured indirectly using a Hess’ cycle.
$3 \mathrm{NO}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \stackrel{\Delta H_{\mathrm{r}}}{\longrightarrow} 2 \mathrm{HNO}_3(\mathrm{l})+\mathrm{NO}(\mathrm{g})$
(a) Explain what is meant by the term enthalpy change of formation.
(b) Complete the Hess’ cycle using the values given in the table and hence calculate the enthalpy change, $\Delta H_r$, for this reaction. Show your working.
$3 \mathrm{NO}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{I}) \stackrel{\Delta H_{\mathrm{r}}}{\longrightarrow} 2 \mathrm{HNO}_3(\mathrm{I})+\mathrm{NO}(\mathrm{g})$
$\Delta H_r=$ $\mathrm{kJmol}^{-1}$ [3]
(c) Nitrogen and oxygen do not react at normal atmospheric temperatures. Explain why.[5]
Nitrogen oxides can be formed naturally in the Earth’s atmosphere from nitrogen and oxygen in the air.
(d) State one way that nitrogen oxides are produced naturally.[1]
(e) Nitrogen dioxide, $\mathrm{NO}_2$, acts as a homogeneous catalyst in the oxidation of atmospheric sulfur dioxide.
(i) Explain why $\mathrm{NO}_2$ is described as a homogeneous catalyst. [3]
(ii) Write equations which describe the two reactions occurring when $\mathrm{NO}_2$ acts as a catalyst in the formation of sulfur trioxide from sulfur dioxide. [2] [Total: 13]
▶️Answer/Explanation
Ans:
(a) M1 (enthalpy / energy change) when one mole of a compound/substance is formed
M2 from its elements in their standard states
(b)
M1 use of correct stoichiometry in calculation $3 x \Delta H_f \mathrm{NO}_2 \quad 1 \mathrm{x}-\Delta \mathrm{H}_f \mathrm{H}_2 \mathrm{O} \quad 2 \mathrm{x} \Delta \mathrm{H}_f \mathrm{HNO}_3 \quad 1 \mathrm{x} \Delta \mathrm{H}_f \mathrm{NO}$
M2 correct signs associated with the appropriate $\Delta H_f$ values/terms used for the calculation of $\Delta \mathrm{H}_{\text {reaction }}$ M3 $\Delta \mathrm{H}_{\text {reaction }}=-(102-286)+(-346+91.1)=-70.9 \mathrm{~kJ} \mathrm{~mol}^{-1}$
(c) M1 nitrogen has a triple bond
M2 EITHER
high energy is needed to break the bond
OR
at normal temperatures there is not enough energy to break the bond / to overcome the activation energy
(d) lightning
(e)(i) M1 define homogeneous
(homogeneous catalyst is) in the same phase / state as the reactants
M2 and M3 Define catalyst
All 3 points scores 2 marks. Any 2 points scores 1 mark
increase the rate
AND
lowers the activation energy
AND
without being chemically altered at the end of the reaction / are regenerated at the end of the reaction
(e)(ii)$\begin{aligned} & \text { M1 NO } \mathrm{N}_2+\mathrm{SO}_2 \rightarrow \mathrm{NO}+\mathrm{SO}_3 \\ & \text { M2 NO }+1 / 2 \mathrm{O}_2 \rightarrow \mathrm{NO}_2\end{aligned}$
Question
The rate of chemical reactions is affected by changes in temperature and pressure.
(a) (i) Draw a curve on the axes to show the Boltzmann distribution of energy of particles in a sample of gaseous krypton atoms at a given temperature.
Label the curve T1 and label the axes.

(ii) On the diagram in (a)(i), draw a second curve to show the distribution of energies of the krypton atoms at a higher temperature.
Label the second curve T2.
(b) The Boltzmann distribution assumes that the particles behave as an ideal gas.
(i) State two assumptions of the kinetic theory as applied to an ideal gas.
(ii) 2.00g of krypton gas, Kr(g), is placed in a sealed 5.00dm³ container at 120°C.
Calculate the pressure, in Pa, of Kr(g) in the container.Assume Kr(g) behaves as an ideal gas.
Show your working.
(iii) State and explain the conditions at which krypton behaves most like an ideal gas.
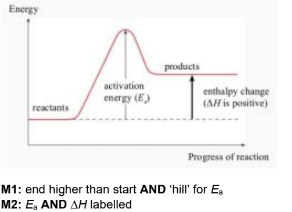
(c) Krypton reacts with fluorine in the presence of ultraviolet light to make krypton difluoride, KrF2(g).
\(Kr(g) + F_{2} (g) \rightarrow KrF_{2}(g)\)
activation energy for the reaction, Ea = +385kJmol-1
enthalpy change of formation of KrF2, ∆Hf = +60.2kJmol-1
(i) Use this information to complete the reaction profile diagram for the formation of KrF2. Label Ea and ∆Hf on the diagram.
Assume the reaction proceeds in one step.
(ii) Explain, in terms of activation energy, Ea, and the collision of particles, how an increase in temperature affects the rate of a chemical reaction.
Answer/Explanation
Answer: (a)(i)
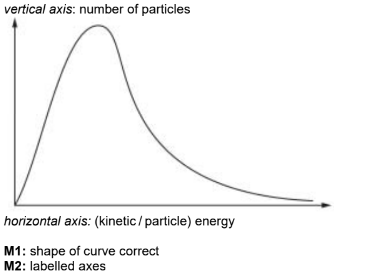
(a)(ii) Labelled line (T2) with lower peak to right of original
(b)(i) Any two from: • no VdW forces present / no forces of attraction between particles
• (ideal gas) particles have no / negligible volume (compared to container)
• collisions between (ideal gas) particles / walls of container are perfectly elastic
• (ideal gas) particles behave as rigid spheres
(b)(ii)

(b)(iii) M1: low pressure AND high temperature
M2: Either of:
• volume of particles is negligible (compared to volume of container)
• VdW forces are insignificant (owing to high kinetic energy of particles)
(c)(i)
(c)(ii) • rate increases • (increase in temperature means) more particles have energy ⩾ activation energy • frequency of successful collisions increases