Question:
Chlorine gas is reacted with aqueous sodium hydroxide. The oxidation number of chlorine changes from 0 to –1 and also from 0 to +1. Under which conditions does this reaction occur and what is the colour of the solid silver salt with chlorine in the oxidation state –1?
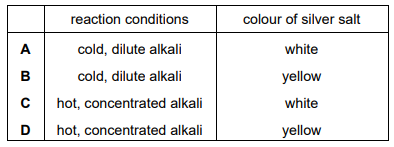
▶️Answer/Explanation
Ans:A
Question:
\(VO_{2}C \imath \) reacts with NaI under acidic conditions.
\(1VO^{2}C \imath~+~2H_{2}SO_{4}~+~2NaI~=~VOSO_{4}~+~I_{2}+~Na_{2}SO_{4}~+~2NH_{2}O\)
The oxidation state of \(C \imath~is~-1~in~VO_{2}C \imath\) and in \(VOC \imath_{2}\). Which row about this reaction is correct?
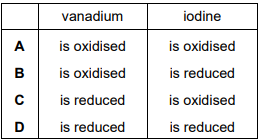
▶️Answer/Explanation
Ans:C
Question
Cobalt can form the positive ion \(Co(NH_{3})_{4}Cl_{2}^{~*}\)
What is the oxidation number of cobalt in this ion?
A +1 B +2 C +3 D +6
▶️Answer/Explanation
Ans:C
Question
In this question you should use changes in oxidation numbers to balance a chemical equation.
Acidified potassium dichromate(VI) solution can oxidise a solution of \(V^{2+}\) ions. The equation for this reaction is shown.
\(aCr_{2}O_{7}^{~2-}~+~bV^{2+}~+~cH^{*}\rightarrow dCr^{3+}+eVO_{3}^{~-}~+~fH_{2}O\)
What is the ratio a :b in the correctly balanced equation?
A 1 : 1 B 1 : 2 C 2 : 1 D 4 : 1
▶️Answer/Explanation
Ans:B
Question:
When the equation is correctly balanced, what is the value of c?
\(aC_{2}H_{4}~+~bH_{2}O~+~cH^{+}~+~2MnO_{4}^{~-}\rightarrow dC_{2}H_{6}O_{2}~+~eMn^{2+}\)
A 3 B 4 C 5 D 6
▶️Answer/Explanation
Ans:D
Question:
In the redox reaction shown, how do the oxidation states of vanadium and sulfur change?
\(VO_{2}^{~+}+SO_{2}\rightarrow V^{3}+SO_{4}^{~2-}\)
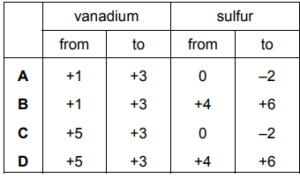
▶️Answer/Explanation
Ans:D
Question
Sodium chloride and sodium iodide react with concentrated sulfuric acid.
Which statements are correct?
1 Sodium chloride is not oxidised by concentrated sulfuric acid.
2 No colour change is seen when concentrated sulfuric acid is added to sodium chloride.
3 Sodium iodide is oxidised by concentrated sulfuric acid.
▶️Answer/Explanation
Ans:A
Question
Nitrogen and phosphorus are both in Group 15 of the Periodic Table. Phosphorus forms a chloride with the formula $\mathrm{PCl} l_5$ but nitrogen does not form $\mathrm{NCl}_5$.
Which statements help to explain this?
1 Nitrogen’s outer shell cannot contain more than eight electrons.
2 Nitrogen cannot have an oxidation state of +5 .
3 Nitrogen is less electronegative than phosphorus.

▶️Answer/Explanation
Ans:D
Question
$ \operatorname{HOCl}(\mathrm{aq})$ is the molecule that kills bacteria when chlorine is added to water.
The following reaction produces this molecule.
$
\mathrm{Cl}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{I}) \rightleftharpoons \mathrm{HOCl}(\mathrm{aq})+\mathrm{H}^{+}(\mathrm{aq})+\mathrm{Cl}^{-}(\mathrm{aq})
$
Which statement about this reaction is correct?
A Chlorine is both oxidised and reduced.
B Chlorine is oxidised but not reduced.
C Hydrogen is both oxidised and reduced.
D Hydrogen is oxidised but not reduced.
▶️Answer/Explanation
Ans:A
Question
Which compound contains two different elements with identical oxidation states?
A $\mathrm{HClO}$
B $\operatorname{Mg}(\mathrm{OH})_2$
C $\mathrm{Na}_2 \mathrm{SO}_4$
D $\mathrm{NH}_4 \mathrm{Cl}$
▶️Answer/Explanation
Ans:A
Question
After black and white photographic film has been developed, unreacted silver bromide is removed by reaction with sodium thiosulfate.
$
\mathrm{AgBr}+2 \mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3 \rightarrow 4 \mathrm{Na}^{+}+\mathrm{Br}^{-}+\left[\mathrm{Ag}\left(\mathrm{S}_2 \mathrm{O}_3\right)_2\right]^{3-}
$
What is the function of the thiosulfate ion?
A to make the silver ions soluble
B to oxidise the silver ions
C to reduce the bromine
D to reduce the silver ions
▶️Answer/Explanation
Ans:A
Question
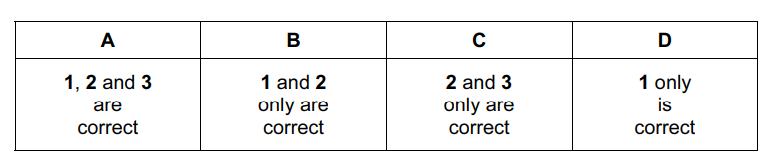
Two half-equations are shown.
\(MnO_4^–+ 2H_2O + 3e^– \rightarrow MnO_2 + 4OH^–\)
\(2OH^– + SO_3^{2–} \rightarrow SO_4^{2–} + H_2O + 2e^–\)
The equation for the reaction between manganate(VII) ions and sulfite ions is shown.
\(uMnO_4^– + vH_2O + wSO_3^{2–} \rightarrow xMnO_2 + ySO_4^{2–} + zOH^–\)
Which statements are correct?
1 u = x = z
2 Manganese is reduced to oxidation state +4.
3 Sulfur is oxidised from oxidation state +4 to +6.
A 1, 2 and 3 B 1 and 2 only C 1 and 3 only D 2 and 3 only
Answer/Explanation
Ans: A
Question
In the treatment of domestic water supplies, chlorine is added to water to kill bacteria. Some \(ClO^–\)
ions are formed.
What is the change in oxidation number of chlorine when forming the \(ClO^–\) ion from aqueous chlorine?
A –1 B 0 C +1 D +2
Answer/Explanation
Ans: C
Question
Solid sodium iodide reacts with concentrated sulfuric acid to form more than one product that contains sulfur.
What is the lowest oxidation number of sulfur in these products?
A –2 B 0 C +4 D +6
Answer/Explanation
Ans: A
Question
When hydrogen iodide is reacted with concentrated sulfuric acid, several reactions occur,including:
\(8HI + H_{2}SO_{4} \rightarrow H_{2}S + 4H_{2}O + 4I_{2}\)
Which row gives the change in oxidation number of iodine and of sulfur in this reaction?
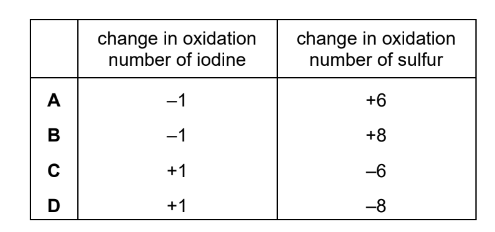
Answer/Explanation
Answer: D
Question
Z is a compound of sodium, chlorine and oxygen. It contains 45.1% by mass of oxygen.
Z is prepared by reacting sodium hydroxide with chlorine.
Which row shows the conditions used for the reaction and the oxidation state of chlorine in Z?
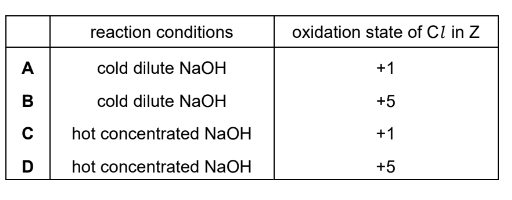
▶️Answer/Explanation
Answer D
Question
Zinc atoms can be oxidised to Zn²+ ions by dichromate(VI) ions in acid solution. Chromium is reduced to Cr³+ in this reaction.
Which equation is correct?
A \(Cr_{2}O_{7}^{2-}+Zn+14H^{+}\rightarrow 2Cr^{3+}+Zn^{2+}+7H_{2}O \)
B \(Cr_{2}O_{7}^{2-}+Zn+14H^{+}\rightarrow 2Cr^{3+}+3Zn^{2+}+7H_{2}O \)
C \(Cr_{2}O_{7}^{2-}+3Zn+14H^{+}\rightarrow 2Cr^{3+}+3Zn^{2+}+7H_{2}O \)
D \(2Cr_{2}O_{7}^{2-}+3Zn+14H^{+}\rightarrow 2Cr^{3+}+3Zn^{2+}+7H_{2}O \)
Answer/Explanation
Answer C
Question
Under standard conditions, which statement is correct?
A Cl2(aq) can oxidise Br–(aq).
B Cl2(aq) can reduce Br–(aq).
C Cl–(aq) can oxidise Br2(aq).
D Cl–(aq) can reduce Br2(aq).
Answer/Explanation
Answer:
A
Question
In which reactions is the underlined element or compound reduced?
1 NaClO + H2O2 → O2 + NaCl + H2O
2 2NH3 + 2Li → 2LiNH2 + H2
3 3CH3CH2OH + K2Cr2O7 + 4H2SO4 → 3CH3CHO + Cr2(SO4)3 + K2SO4 + 7H2O
The responses A to D should be selected on the basis of

Answer/Explanation
Answer:
D
Question
Transition elements and their compounds are widely used as catalysts.
What is the identity and what is the oxidation number of the element present in the catalyst used in the Contact process?
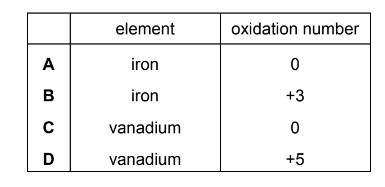
Answer/Explanation
Answer D
Question
Each of the three mixtures shown can result in a chemical reaction.
Which mixtures result in a redox reaction?
1 bromine + hydrogen
2 sodium chloride + concentrated sulfuric acid
3 potassium iodide + silver nitrate
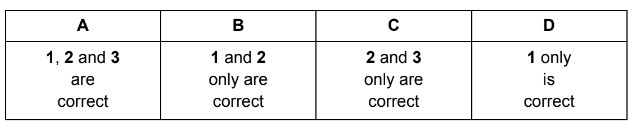
Answer/Explanation
Answer D
Question
Oxidation numbers should be used to answer this question.
A redox reaction takes place between hydroxylammonium ions, [NH3OH]+, and acidified iron(III) ions, Fe3+. The products are iron(II) ions, Fe2+, H+ ions, water and a compound of nitrogen.
The mole ratio of reacting hydroxylammonium ions to reacting iron(III) ions is 1 : 2.
Which nitrogen-containing compound could be formed in the reaction?
A NH3 B N2O C NO D NO2
Answer/Explanation
Answer B
Question
Ethanedioate ions, \(C_{2}O_{4}^{2-}\), react with a suitable reagent to form CO2. A half-equation for this reaction is shown.
\(C_{2}O_{4}^{2-}\rightarrow 2CO_{2}+2e^{-}\)
Which row is correct?
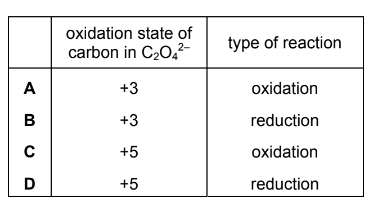
▶️Answer/Explanation
Answer A
Question
Chlorine reacts with hot aqueous sodium hydroxide.
Which oxidation states does chlorine show in the products of this reaction?
1 –1
2 +3
3 +1
The responses A to D should be selected on the basis of
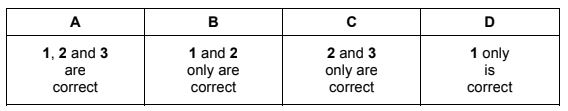
Answer/Explanation
Answer:
D
Question
Ammonia and chlorine react together in the gas phase.
$8 \mathrm{NH}_3+3 \mathrm{Cl}_2 \rightarrow \mathrm{N}_2+6 \mathrm{NH}_4 \mathrm{Cl}$
Which statements are correct?
1 Ammonia behaves as a reducing agent.
2 Ammonia behaves as a base.
3 The oxidation number of hydrogen changes.
The responses A to D should be selected on the basis of

Answer/Explanation
Answer:
B
Question
Bromine is extracted from sea-water. In the final stages of the process two redox reactions take place.
Br2(aq) + SO2(g) + 2H2O(l) → 2HBr(aq) + H2SO4(aq)
2HBr(aq) + Cl2(g) → Br2(g) + 2HCl(aq)
Which row is correct?
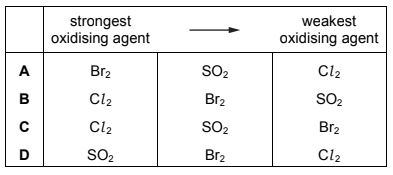
Answer/Explanation
Answer:
B
Question
Which reaction is not a redox reaction?
A Mg + 2HNO3 → Mg(NO3)2 + H2
B 2Mg(NO3)2 → 2MgO + 4NO2 + O2
C SO2 + NO2 → SO3 + NO
D SO3 + H2O → H2SO4
Answer/Explanation
Answer:
D
Question
One way of recovering tin from old printed circuit boards is to dissolve it in a mixture of concentrated hydrochloric acid and concentrated nitric acid. The tin dissolves because it reacts
with the mixture of these concentrated acids.
\(Sn + 4HCl + 2HNO_3 → SnCl_4 + NO_2 + NO + 3H_2O\)
Which statements about this reaction are correct?
1 Nitrogen is present in three different oxidation states in the reactants and products.
2 The oxidation state of tin increases from 0 to +4.
3 The oxidation state of chlorine remains the same.
The responses A to D should be selected on the basis of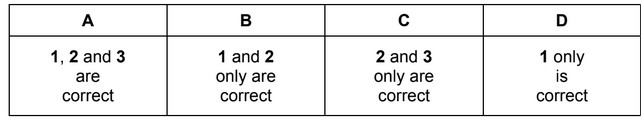
Answer/Explanation
Ans: A
Question
X is either nitrogen or sulfur and forms pollutant oxide Y in a car engine. Further oxidation of Y to Z occurs in the atmosphere. In this further oxidation, 1 mol of Y reacts with 0.5 mol of gaseous oxygen molecules. Which statements about X, Y and Z can be correct?
1 The oxidation number of X increases by two from Y to Z.
2 Y has an unpaired electron in its molecule.
3 Y is a polar molecule.
The responses A to D should be selected on the basis of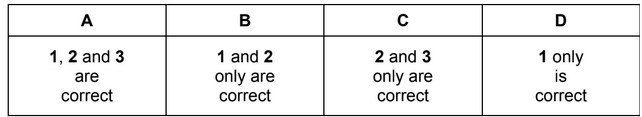
Answer/Explanation
Ans: A
Question
When \(K_2MnO_4\) is dissolved in water, the following reaction occurs.
\(aMnO_4 ^{2–}(aq) + bH_2O(l)\) → \(cMnO_4^–(aq) + dMnO_2(s) + eOH^– (aq)\)
What are the values of a and c in the balanced chemical equation?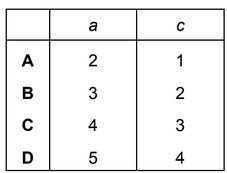
Answer/Explanation
Ans: B
Question
Solutions containing chlorate(I) ions are used as household bleaches and disinfectants. These
solutions decompose on heating as shown.
\(3ClO^–→ ClO_3^–+ 2Cl^–\)
Which oxidation state is shown by chlorine in each of these three ions?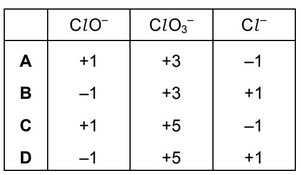
Answer/Explanation
Ans: C
Question
One of the reactions in a lead /acid cell is shown.
\(Pb(s) + PbO_2(s) + 4H^+(aq) + 2SO_4^{2–}(aq) → 2PbSO_4(s) + 2H_2O(l)\)
Which statement about this reaction is correct?
A Lead is both oxidised and reduced.
B Lead is neither oxidised nor reduced.
C Lead is oxidised only.
D Lead is reduced only.
Answer/Explanation
Ans: A
Question
D Only iron is oxidised.
Answer/Explanation
Answer: A
Question
Bromine reacts with water.
Br2 + H2O \(\rightleftharpoons \) HOBr + HBr
Which oxidation states of bromine are present in the equilibrium mixture?
1 +3
2 0
3 –1
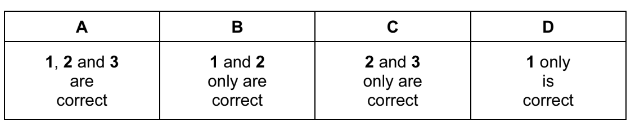
Answer/Explanation
Answer: C
Question
Which pair of reagents will take part in a redox reaction?
A CH3CHCH2 + Br2
B CH3CH2CH2OH + concentrated H3PO4
C CH3COCH3 + HCN
D HCO2C2H5 + dilute H2SO4
Answer/Explanation
Answer: A
Question
In which reaction is the underlined substance acting as a base?
A \( HNO_{3} + H_{2}SO_{4} → H_{2}NO_{3} ^{+} + HSO4^{–}\)
B \(HSiO_{3}^{–}+ HCN → CN^{–} + H_{2}O + SiO_{2}\)
C \(HNO_{2} + HCO_{3}^{–} → H_{2}O + CO_{2} + NO_{2}^{–}\)
D \(C_{6}H_{5}O^{–} + CH_{2}ClCO_{2}H → C_{6}H_{5}OH + CH2ClCO_{2}\)
Answer/Explanation
Ans:C
Question
In oxygen difluoride, OF$_2$, fluorine has an oxidation number of –1. \(OF_{2}\) will react with sulfur dioxide according to the following equation.
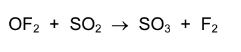
What is oxidised and what is reduced in this reaction?
Answer/Explanation
Ans:C
Question
The salt \( NaClO_{3}\) is used as a non-selective weedkiller. On careful heating, this reaction occurs: \(4NaClO_{3}\) → \(NaCl + 3NaClO_{4}\). On strong heating this reaction occurs: \(NaClO_{4}\) → \(NaCl + 2O_{2}\).
The overall reaction is \(2NaClO_{3}\) → \(2NaCl + 3O_{2}\).
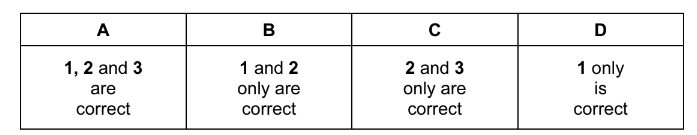
What do these equations show?
1 \(NaClO_{3}\) can behave as an oxidising agent.
2 \( NaClO_{3}\) can behave as a reducing agent.
3 The oxidation numbers of chlorine in the three compounds shown are +6, +8 and –1.
The responses A to D should be selected on the basis of
Answer/Explanation
Ans:B
Question
Element X forms a pollutant oxide Y. Y can be further oxidised to Z. Two students made the following statements.
Student P ‘The molecule of Y contains lone pairs of electrons.’
Student Q ‘The oxidation number of X increases by 1 from Y to Z.’
X could be carbon or nitrogen or sulfur.
Which student(s) made a correct statement?
A P only
B Q only
C both P and Q
D neither P nor Q
Answer/Explanation
Ans:A
Question
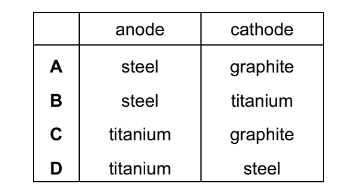
Which row correctly describes the electrodes that can be used in a diaphragm cell for the production of chlorine, hydrogen and sodium hydroxide?
Answer/Explanation
Ans:D
Question
Under standard conditions, which statement is correct?
A\( Cl^{–}\)(aq) can oxidise Br$_2$(aq).
B \( Cl^{–}\) (aq) can reduce\( Br_{2}\)(aq).
C\( Cl_{ 2}\)(aq) can oxidise \(Br^{ –}\) (aq).
D \(Cl_{ 2}\)(aq) can reduce \(Br^{ –}\)(aq).
Answer/Explanation
Answer/Explanation
Ans:C
Question
Chlorine gas reacts with cold aqueous sodium hydroxide. It can also react with hot aqueous sodium hydroxide.
What are the oxidation numbers of chlorine in the products of these reactions?
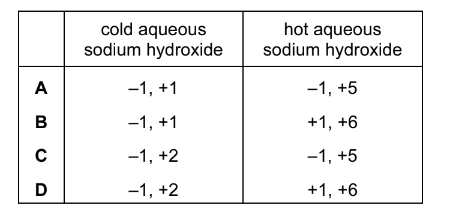
Answer/Explanation
Ans:A
Question
In which reaction does hydrogen behave as an oxidising agent?
A \( H_{2} + Cl_{ 2} → 2HCl\)
B \( C_{2}H_{4} + H_{2} → C_{2}H_{6}\)
C \( N2 + 3H_{2} → 2NH_{3}\)
D \( 2Na + H_{2} → 2NaH\)
Answer/Explanation
Ans:D
Question
Pollutant oxide Y, which contains non-metallic element X, is formed in a car engine. Further oxidation of Y to Z occurs in the atmosphere. In this further oxidation, 1mol of Y reacts with 0.5mol of gaseous oxygen molecules. X could be either nitrogen or sulfur. Which statements about X, Y and Z can be correct?
1 The oxidation number of X increases by two from Y to Z.
2 Y has an unpaired electron in its molecule.
3 Y is a polar molecule.
The responses A to D should be selected on the basis of

Answer/Explanation
Ans:A
Question
The following half reactions occur when potassium iodate(V), \(KIO_3\), in hydrochloric acid solution oxidises iodine to \(ICl_2^–\).
\(IO_3^– + 2Cl^– + 6H^+ + 4e^– → ICl_2^– + 3H_2O\)
\(I_2 + 4Cl^– → 2ICl_2^– + 2e^–\)
What is the ratio of \(IO_3^–\) to \(I_2\) in the balanced chemical equation for the overall reaction?
A 1: 1 B 1 : 2 C 1 : 4 D 2 : 1
Answer/Explanation
Ans: B
Question
Solutions containing chlorate(I) ions are used as household bleaches and disinfectants. These
solutions decompose on heating as shown.
\(3ClO^- \rightarrow Clo_3^- + 2Cl^-\)
Which oxidation state is shown by chlorine in each of these three ions?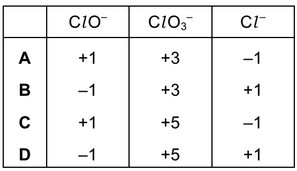
Answer/Explanation
Ans: C
Question
In which reaction does a single nitrogen atom have the greatest change in oxidation number?
A \(4NH_3 + 5O_2 → 4NO + 6H_2O\)
B \(3NO_2 + H_2O → 2HNO_3 + NO\)
C \(2NO + O_2 → 2NO_2\)
D \(4NH_3 + 6NO → 5N_2 + 6H_2O\)
Answer/Explanation
Ans: A
Question
In which reaction does an element undergo the largest change in oxidation state?
- Cl2 + 2OH– → OCl–+ Cl–+ H2O
- 3Cl2 + 6OH– → ClO3–+ 5Cl–+ 3H2O
- Cr2O72– + 6Fe2+ + 14H+ → 2Cr3+ + 6Fe3+ + 7H2O
- 3MnO42– + 4H+ → MnO2 + 2MnO4–+ 2H2O
Answer/Explanation
Ans:
B
Question
Ammonia and chlorine react in the gas phase.
8NH3 + 3Cl2 → N2 + 6NH4Cl
Which statements are correct?
- Ammonia behaves as a reducing agent.
- Ammonia behaves as a base.
- The oxidation number of the hydrogen changes
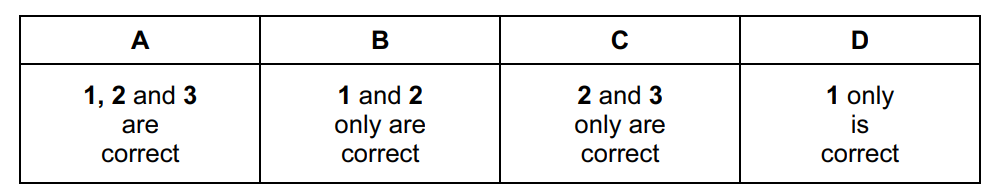
Answer/Explanation
Ans:
B
Question
Carbon monoxide, CO, nitrogen monoxide, NO, and sulfur dioxide, SO2, may all be present in the exhaust fumes from a car engine.
Which reaction concerning these compounds occurs in the atmosphere?
- CO is spontaneously oxidised to CO2
- NO2 is reduced to NO by CO
- NO2 is reduced to NO by SO2
- SO2 is oxidised to SO3 by CO2
Answer/Explanation
Ans:
C
Question
In the treatment of domestic water supplies, chlorine is added to the water to form HClO.
Cl2(aq) + H2O(I) → H+(aq) + Cl–(aq) + HClO(aq)
The HClO reacts further to give ClO–ions.
HClO(aq) + H2O(I) → H3O+(aq) + ClO–(aq)
Both HClO and ClO–kill bacteria by oxidation.
What is the overall change in oxidation number of chlorine when forming the Cl O–ion from the aqueous chlorine?
A -1 B 0 C +1 D +2
Answer/Explanation
Ans:
C
Question
The diagram shows a cell for the manufacture of aluminium.
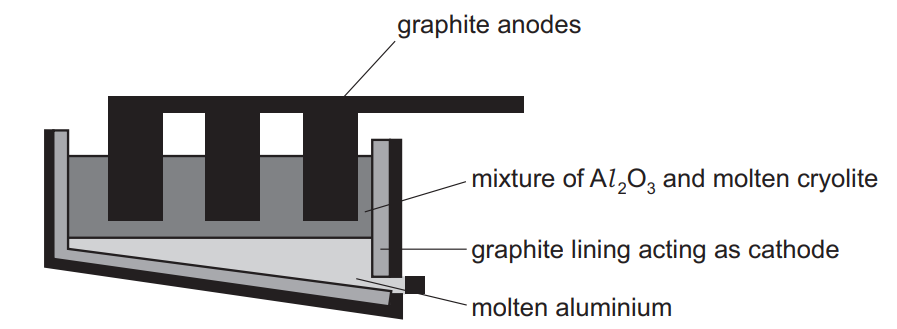
Which statement is incorrect?
- Aluminium ions are oxidised in this process.
- Aluminium is liberated at the cathode by the reaction Al 3+ + 3e– → Al.
- The cryolite acts as a solvent.
- The graphite anode burns away.
Answer/Explanation
Ans:
A
Question
The oxide of titanium, TiO2, is used as a ‘whitener’ in toothpaste. It is obtained from the ore iron(II) titanate, FeTiO3.
What is the change, if any, in the oxidation number (oxidation state) of titanium in the reaction FeTiO3 → TiO2?
- It is oxidised from +3 to +4.
- It is reduced from +3 to +2.
- It is reduced from +6 to +4.
- There is no change in the oxidation number.
Answer/Explanation
Ans:
D