Question
The units of $K_{\mathrm{c}}$ for an equilibrium reaction are $\mathrm{mol}^{-1} \mathrm{dm}^3$.
What could be the equation for the equilibrium?
$1 \mathrm{~A}(\mathrm{aq})+\mathrm{B}(\mathrm{aq}) \rightleftharpoons \mathrm{C}(\mathrm{s})+\mathrm{D}(\mathrm{aq})$
$2 \mathrm{P}(\mathrm{aq})+\mathrm{Q}(\mathrm{aq}) \rightleftharpoons \mathrm{R}(\mathrm{aq})$
$3 \mathrm{~W}(\mathrm{aq})+2 \mathrm{X}(\mathrm{aq}) \rightleftharpoons \mathrm{Y}(\mathrm{aq})+\mathrm{Z}(\mathrm{aq})$
▶️Answer/Explanation
Ans:A
Question
In aqueous solution, sulfuric acid dissociates as shown.
$
\begin{array}{ll}
\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{HSO}_4^{-}+\mathrm{H}^{+} & \text {This reaction goes to completion. } \\
\mathrm{HSO}_4^{-} \rightleftharpoons \mathrm{SO}_4^{2-}+\mathrm{H}^{+} & \text {This reaction reaches equilibrium with constant } K_{\mathrm{c}} \text {. }
\end{array}
$
Analysis of a $2.00 \mathrm{moldm}^{-3}$ solution of $\mathrm{H}_2 \mathrm{SO}_4$ found the $\mathrm{HSO}_4^{-}$concentration to be $1.988 \mathrm{~mol} \mathrm{dm}^{-3}$.
What is $K_c$ ?
A $1.381 \times 10^5 \mathrm{dm}^3 \mathrm{~mol}^{-1}$
B $\quad 82.34 \mathrm{dm}^3 \mathrm{~mol}^{-1}$
C $1.214 \times 10^{-2} \mathrm{~mol} \mathrm{dm}^{-3}$
D $7.244 \times 10^{-5} \mathrm{~mol} \mathrm{dm}^{-3}$
▶️Answer/Explanation
Ans:C
Question
What are the units of \(K_{p}\) for the reaction shown?
\(H_{2}O(g)~+~C(s)\rightleftharpoons H_{2}(g)~+~CO(g)\)
A \(Pa^{-1}\) B Pa C Pa^{2} D no units
▶️Answer/Explanation
Ans:B
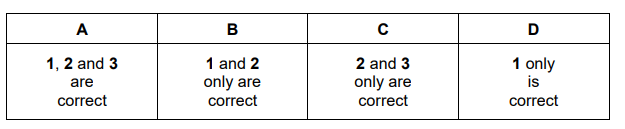
Question
Ethene can be oxidised to form epoxyethane, \( C_{2}H_{4}O. \)
\(C_{2}H_{4}(g)~+~\frac{1}{2}O_{2}(g)\rightleftharpoons C_{2}H_{4}(g)~~~\Delta ^{\theta}=107kJ~mol^{-1}\)
Which set of conditions gives the greatest yield of epoxyethane at equilibrium?
▶️Answer/Explanation
Ans:A
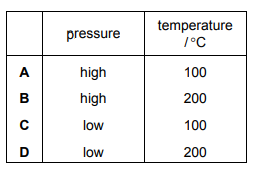
Question:
The main stage in the Contact process is an equilibrium reaction.
\(2SO_{2}~+~O_{2}\rightleftharpoons 2SO_{3}\)
Which row describes the effect of the named condition on the equilibrium yield?
▶️Answer/Explanation
Ans:B
Question:
The equation for the reaction between silver chloride and aqueous ammonia is shown.
\(AgC \imath(s)+2NH_{3}(aq)\rightleftharpoons [ag(NH_{3})_{2}]^{+}(aq)~+~C\imath^{-}(aq)\)
What are the units of \(K_{c}\) for this reaction?
A no units B \(mol^{-1}dm^{3}\) C \(mol^dm^{3}\) D \(mol^{2}dm^{-6}\)
▶️Answer/Explanation
Ans: A
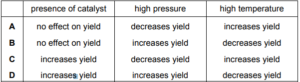
Question
Sulfur dioxide and oxygen react in the gas phase.
$
2 \mathrm{SO}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g}) \rightleftharpoons 2 \mathrm{SO}_3(\mathrm{~g}) \quad \Delta H=-197 \mathrm{~kJ} \mathrm{~mol}^{-1}
$
Which statements are correct?
1 Increasing the pressure increases the equilibrium yield of $\mathrm{SO}_3$.
2 Increasing the temperature lowers the value of the equilibrium constant $K_{\mathrm{p}}$.
3 The presence of a vanadium(V) oxide catalyst increases the equilibrium yield of $\mathrm{SO}_3$.
▶️Answer/Explanation
Ans:B
Question
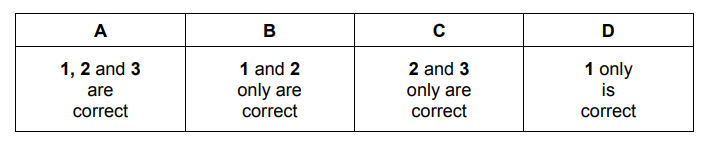
Which statements about reversible reactions are correct?
1 An increase in concentration of a reactant always increases the concentration of the product.
2 An increase in temperature always increases the rate at which the equilibrium is established.
3 An increase in temperature always increases the concentration of the product at equilibrium
▶️Answer/Explanation
Ans:B
Question
What is the volume of steam produced when $1.00 \mathrm{~g}$ of ice is heated to $323^{\circ} \mathrm{C}$ at a pressure of $101 \mathrm{kPa}$ ?
A $0.27 \mathrm{dm}^3$
B $1.3 \mathrm{dm}^3$
C $2.7 \mathrm{dm}^3$
D $48 \mathrm{dm}^3$
▶️Answer/Explanation
Ans:C
Question
In an experiment, $2.00 \mathrm{~mol}$ of hydrogen and $3.00 \mathrm{~mol}$ of iodine were heated together in a sealed container and allowed to reach equilibrium at a fixed temperature. The container had a fixed volume of $1.00 \mathrm{dm}^3$. At equilibrium, there were $2.40 \mathrm{~mol}$ of iodine present in the mixture.
$
\mathrm{H}_2(\mathrm{~g})+\mathrm{I}_2(\mathrm{~g}) \rightleftharpoons 2 \mathrm{HI}(\mathrm{g})
$
What is the value of the equilibrium constant, $K_c$ ?
A 0.107
B 0.357
C 0.429
D 2.33
▶️Answer/Explanation
Ans:C
Question
Hydrogen and iodine react to form hydrogen iodide in an exothermic reaction. The equation is shown.
\(H_2(g) + I_2(g) \leftrightarrow 2HI(g)\)
\(A~ 1 m^3\) reaction vessel contains \(H_2\), \(I_2\) and HI gases at equilibrium. The temperature is changed such that the total pressure in the 1 \(m^3\) vessel doubles.
What is the effect on the value of \(K_p\) and on the position of equilibrium?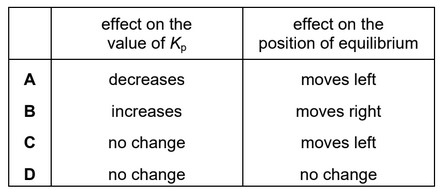
Answer/Explanation
Ans: A
Question
Ethanoic acid is mixed with ethanol.
The ethanol is contaminated with a small amount of methanol.
The following equilibria are established.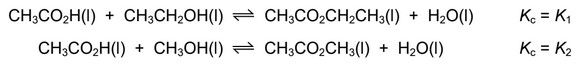
Which statement about the equilibrium mixture is correct?
A Only ethyl ethanoate will be formed because there is much more ethanol present than methanol.
B In this mixture \(\frac{[CH_3CO_2CH_2CH_3]}{[CH_3CO_2CH_3]}=\frac{K_1}{K_2}\)
C Adding water to the mixture will alter the mole ratio of the two esters.
D Adding methyl ethanoate to the mixture will increase the number of moles of ethyl ethanoate.
Answer/Explanation
Ans: D
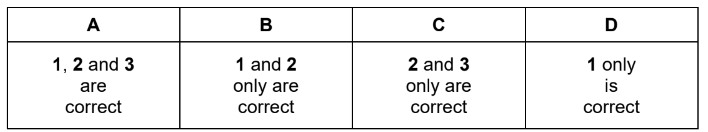
Question
Iron reacts with steam to produce hydrogen and an oxide of iron.
\(3Fe(s)+4H_{2O(g)}\leftrightharpoons Fe_{3}O_{4}(s)+4H_{2}(g)\)
A system containing all four substances is at equilibrium.
Which changes will decrease the mass of Fe present at equilibrium?
1 addition of steam at constant pressure
2 increase in overall pressure
3 addition of an effective catalyst
▶️Answer/Explanation
Answer D
Question
The equation shows that H2(g) and I2(g) react to form an equilibrium mixture.
\(H_{2}(g)+I_{_{2}}(g)\rightleftharpoons 2HI(g)\Delta H^{\circ}=-9.6kJmol^{-1}\)
A mixture containing equal amounts of H2(g) and I2(g) is made at temperature T1 and the composition of the mixture is monitored. A graph of the results is shown.
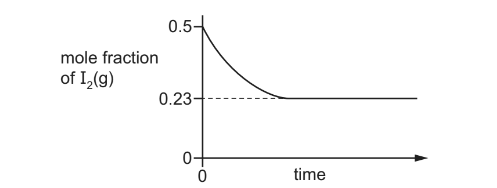
The experiment is repeated at a lower temperature, T2.
Which new graph would be obtained?
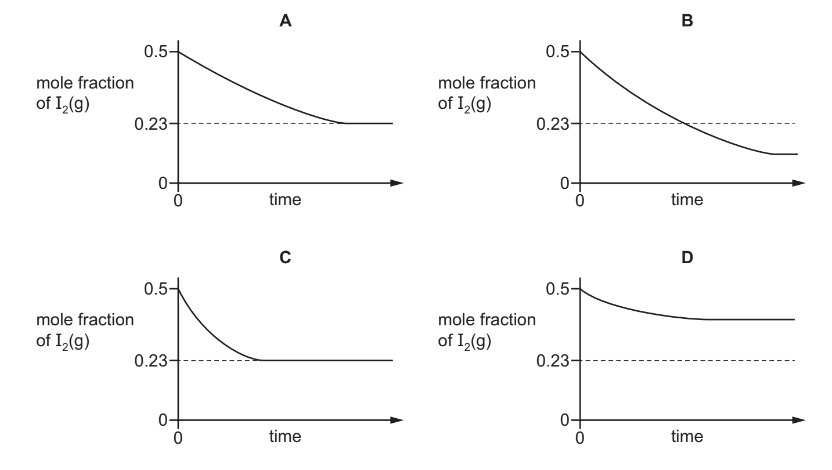
▶️Answer/Explanation
Answer B
Question
When copper is added to a solution of silver ions, the following equilibrium is established.
Cu(s) + 2Ag+ (aq) \(\leftrightharpoons \) Cu2+(aq) + 2Ag(s) Kc = 1.0 × 105
What is the concentration of silver ions at equilibrium when [Cu2+] = 0.10mol dm–3?
A 5.0 × 10–7 mol dm–3
B 5.0 × 10–4 mol dm–3
C 1.0 × 10–3 mol dm–3
D 1.0 × 102 mol dm–3
Answer/Explanation
Answer:
C
Question
The reaction between sulfur dioxide and oxygen is reversible.
\(2SO_{2}(g) + O2(g) \rightleftharpoons 2SO_{3}(g)\) Kc = 280 mol-1 dm³ at 1000K
In an equilibrium mixture at 1000K the sulfur dioxide concentration is 0.200 moldm-3 and the oxygen concentration is 0.100 mol dm-3.
What is the sulfur trioxide concentration?
A 1.058 mol dm-3
B 1.120 mol dm-3
C 2.366 mol dm-3
D 5.600 mol dm-3
Answer/Explanation
Answer A
Question
The reaction between sulfur dioxide and oxygen is reversible.
2SO2(g) + O2(g) \(\leftrightharpoons \) 2SO3(g) Kc = 280 mol–1 dm3 at 1000K
In an equilibrium mixture at 1000K the sulfur trioxide concentration is 6.00mol dm–3. The sulfur dioxide concentration is twice the oxygen concentration.
What is the sulfur dioxide concentration?
A 0.175 mol dm–3
B 0.254 mol dm–3
C 0.318 mol dm–3
D 0.636 mol dm–3
Answer/Explanation
Answer D
Question
Hydrogen iodide gas decomposes reversibly producing iodine vapour and hydrogen.
2HI(g) \(\leftrightharpoons \) I2(g) + H2(g) ∆H = +12 kJ mol–1
The position of the equilibrium for this reaction may be altered by changing the external conditions.
Which row correctly describes the change in position of equilibrium?
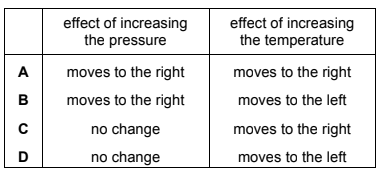
Answer/Explanation
Answer C
Question
0.200 mol of sulfur dioxide and 0.200 mol of oxygen are placed in a 1.00 dm3 sealed container. The gases are allowed to react until equilibrium is reached.
2SO2 + O2 \(\rightleftharpoons \) 2SO3
At equilibrium there is 0.100 mol of SO3 in the container.
What is the value of Kc?
A 0.150 mol dm–3
B 0.800 mol dm–3
C 1.25 mol–1 dm3
D 6.67 mol–1 dm3
Answer/Explanation
Answer D
Question
Which statements are correct when a reversible reaction is at equilibrium?
1 All species are at equal concentration.
2 The concentrations of all species remain constant.
3 The rate of the forward reaction equals the rate of the reverse reaction.
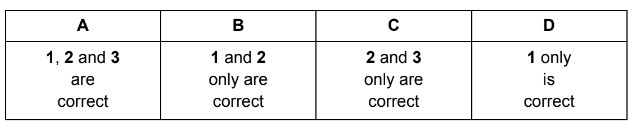
Answer/Explanation
Answer C
Question
The reaction between sulfur dioxide and oxygen is reversible.
\[
\begin{aligned}
& 2 \mathrm{SO}_2+\mathrm{O}_2 \rightleftharpoons 2 \mathrm{SO}_3 \\
& \Delta \mathrm{H}^{\oplus}=-196 \mathrm{~kJ} \mathrm{~mol}^{-1}
\end{aligned}
\]
Which conditions of pressure and temperature favour the reverse reaction?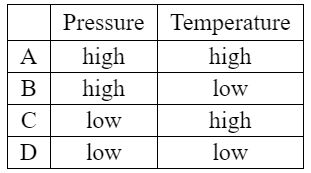
Answer/Explanation
Answer:
C
Question
Hydrogen iodide dissociates into hydrogen and iodine.
\(2HI(g) \leftrightarrow H_2(g) + I_2(g)\)
In an experiment, b mol of hydrogen iodide were put into a sealed vessel at pressure p. At equilibrium, x mol of the hydrogen iodide had dissociated.
Which expression for \(K_p\) is correct?
A \(\frac{x^2}{(b-x)^2}\) B \(\frac{x^2p^2}{(b-x)^2}\) C \(\frac{x^2p^2}{4b(b-x)}\) D \(\frac{x^2}{4(b-x)^2}\)
Answer/Explanation
Ans: D
Question
Which changes can be used to measure the rates of chemical reactions?
1 the decrease in concentration of a reactant per unit time
2 the rate of appearance of a product
3 the increase in total volume per unit time at constant pressure
The responses A to D should be selected on the basis of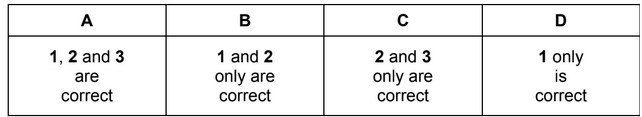
Answer/Explanation
Ans: A
Question
Methanol can be produced from hydrogen and carbon monoxide.
\(2H_2(g) + CO(g) \leftrightarrow CH_3OH(g)\)
What is the expression for \(K_p\) for this reaction?
A \(K_p = \frac{(2_{H_2})^2 \times P_{CO}}{P_{CH_3OH}}\)
B \(K_p = \frac{(P_{H_2})^2 \times P_{CO}}{P_{CH_3OH}}\)
C \(K_p = \frac{P_{CH_3OH}}{(P_{H_2})^2 \times P_{CO}}\)
D \(K_P = \frac{P_{CH_3OH}}{P_{CO} \times (2P_{H_2})^2}\)
Answer/Explanation
Ans: C
Question
In the manufacture of sulfuric acid, the following exothermic reaction occurs.
\(2SO_{2}(g) + O_{2}(g) \rightleftharpoons 2SO_{3}(g)\)
Which changes will move the position of the equilibrium to the right?
1 increasing the pressure
2 increasing the temperature
3 using twice as much catalyst
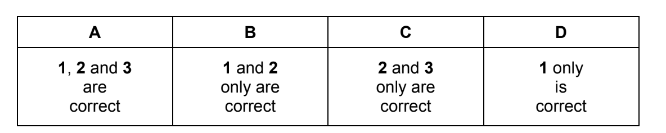
Answer/Explanation
Answer: D
Question
The equation for the reaction between carbon monoxide and hydrogen is shown.
\(CO(g)+3H_{2}(g)\rightleftharpoons CH_{4}(g)+H_{2}O(g)\)
What are the units of Kp for this reaction?
A kPa B kPa–1 C kPa2 D kPa–2
Answer/Explanation
Answer: D
Question
An aqueous solution was prepared containing a mixture of 1.0mol of AgNO3 and 1.0mol of FeSO4 in 1.00dm3 of water. When equilibrium was established, there was 0.44mol of Ag+ (aq) in the mixture.
\(Ag^{+}(aq)+Fe^{2+}(aq)\rightleftharpoons Ag(s)+Fe^{3+}(aq)\)
What is the numerical value of Kc?
A 0.62 B 1.40 C 1.62 D 2.89
Answer/Explanation
Answer: D
Question
One molecule of haemoglobin, Hb, can bind with four molecules of oxygen according to the following equation. When the equilibrium concentration of O2 is \(7.6 × 10^{–6 }moldm^{–3}\), the equilibrium concentrations of Hb and\( Hb(O_{2})_{4}\) are equal.
What is the value of \(K_{c}\) for this equilibrium?
A \(3.0 × 10^{20}\)
B \(1.3 × 10^{5}\)
C \(7.6 × 10^{–6}\)
D \(3.3 × 10^{–21}\)
Answer/Explanation
Ans:A
Question
One mole of phosphorus(V) chloride, \(PCl _{5}\), is heated to 600K in a sealed flask of volume\( 1dm^{3}\). Equilibrium is established and measurements are taken.
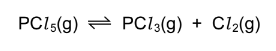
The experiment is repeated with one mole of phosphorus(V) chloride heated to 600K in a sealed
flask of volume\( 2dm^{3}\) . How will the measurements vary?
A The equilibrium concentrations of\( PCl _{3}\)(g) and\( Cl_{ 2}\)(g) are higher in the second experiment.
B The equilibrium concentration of \(PCl _{5}\)(g) is lower in the second experiment.
C The equilibrium concentrations of all three gases are the same in both experiments.
D The value of the equilibrium constant is higher in the second experiment.
Answer/Explanation
Ans:B
Question
A mixture of nitrogen and hydrogen gases, at a temperature of 500K, was put into an evacuated
vessel of volume \(6.0dm^{3}\) . The vessel was then sealed. \( N_{2}(g) + 3H_{2}(g) \rightleftharpoons 2NH_{3}\)(g)
The mixture was allowed to reach equilibrium. It was found that 7.2mol of \(N_{2}\) and 12.0mol of \(H_{2}\) were present in the equilibrium mixture. The value of the equilibrium constant,\( K_{c}\), for this
equilibrium is \(6.0 × 10^{–2}\) at 500K. What is the concentration of ammonia present in the equilibrium mixture at 500K?
A \(0.58moldm^{–3}\)
B \( 0.76moldm^{–3}\)
C \(3.5moldm^{–3}\)
D \(27moldm^{–3}\)
Answer/Explanation
Ans:B
Question
What are necessary properties of a dynamic equilibrium?
1 Equal amounts of reactants and products are present.
2 Concentrations of reactants and products remain constant.
3 The rate of the forward reaction is the same as the rate of the reverse reaction.
The responses A to D should be selected on the basis of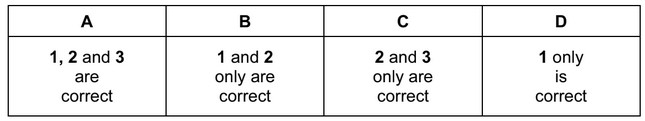
No other combination of statements is used as a correct response.
Answer/Explanation
Ans: C
Question
Nitrogen reacts with hydrogen to produce ammonia.
\(N_2(g) + 3H_2(g) \rightleftarrow 2NH_3(g)\)
A mixture of 2.00 mol of nitrogen, 6.00mol of hydrogen, and 2.40mol of ammonia is allowed to
reach equilibrium in a sealed vessel of volume 1 \(dm^3\) under certain conditions. It was found that 2.32 mol of nitrogen were present in the equilibrium mixture.
What is the value of Kc under these conditions?
A \(\frac{(1.76)^2}{(2.32)(6.96)^3}\)
B \(\frac{(1.76)^2}{(2.32)(6.32)^3}
C \(\frac{(2.08)^2}{(2.32)(6.32)^3}\)
D \(\frac{(2.40)^2}{(2.32)(6.00)^3}\)
Answer/Explanation
Ans: A
Question
Two moles of compound P were placed in a vessel. The compound P was partly decomposed by heating. A dynamic equilibrium between chemicals P, Q and R was established.
At equilibrium, x mol of R were present and the total number of moles present was (2 + x).
What is the equation for this equilibrium?
- P \(\rightleftharpoons \) 2Q + R
- 2P \(\rightleftharpoons \) 2Q + R
- 2P \(\rightleftharpoons \) Q + R
- 2P \(\rightleftharpoons \) Q + 2R
Answer/Explanation
Ans:
B
Question
Ammonia is manufactured by the Haber Process, in an exothermic reaction.
Assuming that the amount of catalyst remains constant, which change will not bring about an increase in the rate of the forward reaction?
- decreasing the size of the catalyst pieces
- increasing the pressure
- increasing the temperature
- removing the ammonia as it is formed
Answer/Explanation
Ans:
D