Question
Calcium, magnesium and radium are Group 2 elements. Radium follows the same trends as the other members of Group 2.
(a) Identify the highest energy orbital which contains electrons in a calcium atom. Sketch the shape of this orbital.
identity of highest energy orbital in Ca …………………………shape
(b) (i) Write the equation for the thermal decomposition of calcium nitrate.
(ii) Suggest which of the Group 2 nitrates, calcium, magnesium or radium, requires the highest temperature to decompose. Explain your answer.
(c) Predict what you would observe when aqueous radium chloride is added to aqueous sodium sulfate.
Do not refer to temperature changes in your answer.
(d) (i) \(^{25}_{12}\) Mg is an isotope of magnesium.
Determine the number of protons and neutrons in an atom of \(^{12}_{25}Mg\).
number of protons ………………………………………………………………………………………………..
number of neutrons ………………………………………………………………………………………………
(ii) State the full electronic configuration of an atom of \(^{12}_{25}Mg\).
(e) A sample of magnesium contains three isotopes, \(^{25}\)Mg, \(^{26}\)Mg and X. The percentage abundance of the three isotopes is shown in Table 1.1.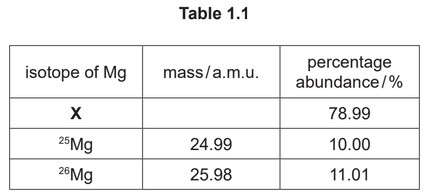
(i) The relative atomic mass, \(A_r\) , is calculated by comparing the average mass of the isotopes of an element to the unified atomic mass unit.
Define the unified atomic mass unit.
(ii) Calculate the mass of X. Use data from Table 1.1 and \(A_r\) (magnesium) = 24.31 in your calculation. Show your working.
mass of X = ………
(iii) State one similarity and one difference in the properties of these isotopes of magnesium.Explain your answer.
(f) Magnesium, Mg, burns in oxygen, \(O_2\).
The activation energy, \(E_a\), for this reaction is +148kJ\(mol^{–1}\).
(i) State one observation when magnesium burns in oxygen.Do not refer to temperature changes in your answer.
(ii) On Fig. 1.1:
● sketch a reaction pathway diagram for the reaction that occurs when Mg burns in \(O_2\)
● label the diagram to show the enthalpy change, ∆H, and the activation energy, \(E_a\), for
the reaction.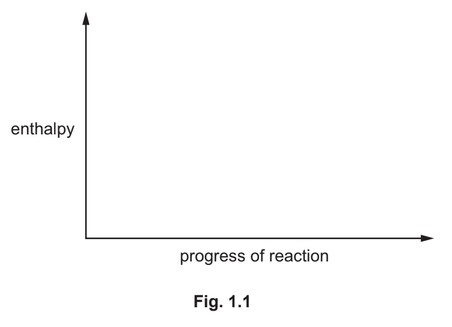
(g) Cold water reacts slowly with a piece of Mg to produce bubbles of \(H_2(g)\). Cold water reacts rapidly with burning Mg to produce \(H_2(g)\) in an explosive mixture.
\(Mg + 2H_2O \rightarrow Mg(OH)_2 + H_2\)
Explain why the rate of reaction of cold water with burning magnesium is greater.
Answer/Explanation
Answer:
(a) Identify and draw the shape of highest energy orbital of Ca 4s AND ![]()
(b) (i) \(Ca(NO_3)_2 \rightarrow CaO + 2NO_2 + 1/2O_2\)
(ii) radium (nitrate) as thermal stability increases down group / has the greatest thermal stability
(c) white precipitate / solid (of radium sulfate)
(d) (i) number of protons: 12
number of neutrons: 13
(ii) \(1s^22s^22p^63s^2\)
(e) (i) 1 / 12 (one twelfth) the mass of a carbon-12 / \(^{12}\)C atom
(ii) M1 correct expression relating \(A_r\) to the mass / % abundance of the three isotopes
24.31 = x \times 0.7899 + 24.99 \rightarrow 0.1000 + 25.98 \times 0.1101\)
M2 correct answer to 4 sig figs
atomic mass of X = 23.99
(iii) M1 (magnesium isotopes have) identical chemical properties
AND same electron(ic) arrangement / configuration
M2 different physical properties AND different number of neutrons
(f) (i) white flame / light OR white solid / smoke
(ii) M1 sketch shows exothermic reaction with a ‘hump’ AND labelled reactants \((Mg + O_2)\) and products (MgO)
M2 arrow from reactants / \(Mg + O_2\) to products / MgO shown as ∆H
M3 arrow showing activation energy / \(E_a\) / (+)148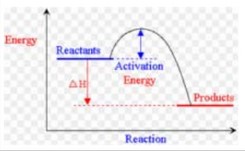
(g) M1 (heat / energy released from burning Mg) provides more particles with energy ⩾ \(E_a\)
M2 frequency of successful / effective collisions is greater