Question
(a) The oxidation of nitrogen(II) oxide is shown in the equation.
$
2 \mathrm{NO}(\mathrm{g})+\mathrm{O}_2(\mathrm{~g}) \rightarrow 2 \mathrm{NO}_2(\mathrm{~g})
$
The initial rate of this reaction was measured, starting with different concentrations of the two reactants. The following results were obtained.
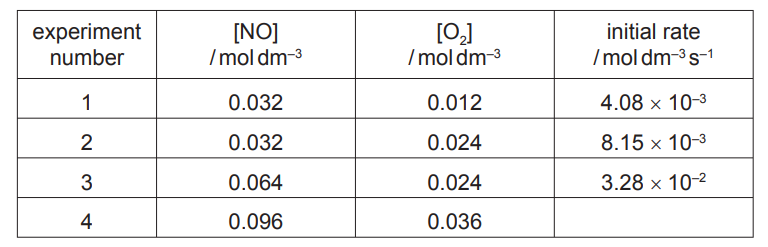
(i) Use the data in the table to determine the order with respect to each reactant. Show your reasoning.
(ii) Calculate the initial rate in experiment 4. Give your answer to two significant figures.
initial rate $=$ $\mathrm{moldm}^{-3} \mathrm{~s}^{-1}$
(iii) Write the rate equation for this reaction.
(iv) Use the results of experiment 1 to calculate the rate constant, $k$, for this reaction. Include the units of $k$.
rate constant, $k=$ units $[6]$
(b) (i) On the following axes
- draw two Boltzmann distribution curves, at two different temperatures, $T_1$ and $T_2\left(T_2>T_1\right)$,
- label the curves and the axes.

(ii) State and explain, using your diagram, the effect of increasing temperature on the rate of reaction. [5]
(c) The compound nitrosyl fluoride, NOF, can be formed by the following reaction.
$
2 \mathrm{NO}(\mathrm{g})+\mathrm{F}_2(\mathrm{~g}) \rightleftharpoons 2 \mathrm{NOF}(\mathrm{g})
$
The rate is first order with respect to $\mathrm{NO}$ and $\mathrm{F}_2$. The reaction mechanism has two steps.
Suggest equations for the two steps of this mechanism, stating which is the rate determining slower step. [2] [Total: 13]
▶️Answer/Explanation
Ans:
(a) (i) $[\mathrm{NO}] 2^{\text {nd }}$ order and the concentration is $\times 2$, rate $\times 4$
$\left[\mathrm{O}_2\right] \quad 1^{\text {st }}$ order and evidence of using expt $1 \& 2$ when the concentration is $\times 2$, rate doubles
(ii) $\quad(0.00408 \times 27)$
rate $=\underline{0.11}\left(\mathrm{~mol} \mathrm{dm}^{-3} \mathrm{~s}^{-1}\right)$ to $2 \mathbf{s f}$
(iii) (Rate $=) k\left[\mathrm{O}_2\right][\mathrm{NO}]^2$
(iv) $k=332(.03125)$
$\mathrm{mol}^{-2} \mathrm{dm}^6 \mathrm{~s}^{-1}$
(b) (i) labelled axes $x$-axis: energy (KE) and $y$-axis: molecules or particles two curves: starts origin; not touching $x$-axis again; no levelling out; curves only intersecting once curves labelled and $\mathrm{T} 2$ is to the right and lower max than T1
(ii) rate increases and energy of the particles increases more particles have $E_{\mathrm{a}}$
(c) 1 mole of $F_2$ and 1 mole NO reacting in the slow step a balanced mechanism consistent with overall equation
$
\begin{aligned}
& \mathrm{F}_2+\mathrm{NO} \rightarrow \mathrm{NOF}+\mathrm{F} \\
& \mathrm{NO}+\mathrm{F} \rightarrow \mathrm{NOF}
\end{aligned}
$
OR
$
\begin{aligned}
& \mathrm{F}_2+\mathrm{NO} \rightarrow \mathrm{NOF}_2 \\
& \mathrm{NO}+\mathrm{NOF}_2 \rightarrow 2 \mathrm{NOF}
\end{aligned}
$
Question
The elements in Group 2, and their compounds, show many similarities and trends in their properties.
(a) Magnesium, calcium, strontium and barium all react with cold water.
(i) Describe what you would see when some calcium is added to cold water.[3]
(ii) Write an equation for the reaction taking place in (i).[1]
(iii) Describe how the reaction of barium with cold water would differ from the reaction of calcium in (i) in terms of what you would see.[1]
(b) Magnesium oxide can be formed by the reaction of magnesium and oxygen in the air.
(i) Draw a fully labelled reaction pathway diagram for the reaction between magnesium and oxygen.
(ii) Explain why there is no visible reaction when a piece of magnesium ribbon is exposed to the air
(iii) Magnesium oxide is used to manufacture heat-resistant bricks for furnace linings in the steel-making industry.
State and explain the property of magnesium oxide that makes it suitable for this use.[2]
(iv) Suggest a reason why magnesium oxide cannot be used as a lining for any furnaces containing acidic materials.[1]
(c) The nitrates and carbonates of the Group 2 elements, from magnesium to barium, decompose when heated.
(i) State the trend in the temperature of thermal decomposition of these Group 2 nitrates and carbonates.[1]
(ii) Give the equation for the thermal decomposition of magnesium carbonate.[1]
(iii) Give the equation for the thermal decomposition of calcium nitrate.[1][Total: 15]
▶️Answer/Explanation
Ans:
(a) (i) bubbles / effervescence/ fizzing
calcium gets smaller/ disappears
water turns cloudy / milky
calcium sinks
(ii) $\quad \mathrm{Ca}+2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Ca}(\mathrm{OH})_2+\mathrm{H}_2$
(iii) faster bubbling/ disappearance of Ba
OR
no/ less precipitate forms (owtte)
(b) (i)
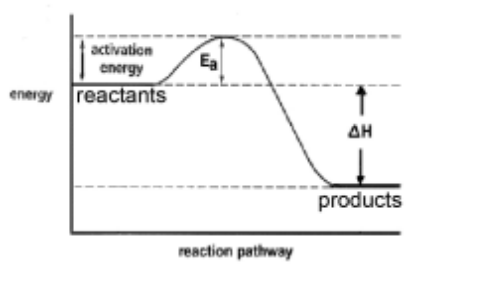
M1 – general layout with products below reactants AND both labelled M2 $-E_a$ and $\Delta H /$ energy change/released labelled with vertical lines
(ii) activation energy is high so few/no particles with $E \geqslant E_{\mathrm{a}}$
(iii) high melting/ boiling point
strong forces (of attraction/between oppositely charged ions)/ strong (ionic) bonding
(iv) MgO is basic / reacts with acid
(c) (i) increases (down the group)
(ii) $\mathrm{MgCO}_3 \rightarrow \mathrm{MgO}+\mathrm{CO}_2$
(iii) $2 \mathrm{Ca}\left(\mathrm{NO}_3\right)_2 \rightarrow 2 \mathrm{CaO}+4 \mathrm{NO}_2+\mathrm{O}_2$