Question
Nitric acid, \(HNO_{3}\) can be made by reacting nitrogen dioxide with water.
The enthalpy change for the reaction can be measured indirectly using a Hess’ cycle.
$3 \mathrm{NO}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \stackrel{\Delta H_{\mathrm{r}}}{\longrightarrow} 2 \mathrm{HNO}_3(\mathrm{l})+\mathrm{NO}(\mathrm{g})$
(a) Explain what is meant by the term enthalpy change of formation.
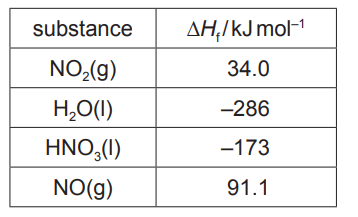
(b) Complete the Hess’ cycle using the values given in the table and hence calculate the enthalpy change, $\Delta H_r$, for this reaction. Show your working.
$3 \mathrm{NO}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{I}) \stackrel{\Delta H_{\mathrm{r}}}{\longrightarrow} 2 \mathrm{HNO}_3(\mathrm{I})+\mathrm{NO}(\mathrm{g})$
$\Delta H_r=$ $\mathrm{kJmol}^{-1}$ [3]
(c) Nitrogen and oxygen do not react at normal atmospheric temperatures. Explain why.[5]
Nitrogen oxides can be formed naturally in the Earth’s atmosphere from nitrogen and oxygen in the air.
(d) State one way that nitrogen oxides are produced naturally.[1]
(e) Nitrogen dioxide, $\mathrm{NO}_2$, acts as a homogeneous catalyst in the oxidation of atmospheric sulfur dioxide.
(i) Explain why $\mathrm{NO}_2$ is described as a homogeneous catalyst. [3]
(ii) Write equations which describe the two reactions occurring when $\mathrm{NO}_2$ acts as a catalyst in the formation of sulfur trioxide from sulfur dioxide. [2] [Total: 13]
▶️Answer/Explanation
Ans:
(a) M1 (enthalpy / energy change) when one mole of a compound/substance is formed
M2 from its elements in their standard states
(b)
M1 use of correct stoichiometry in calculation $3 x \Delta H_f \mathrm{NO}_2 \quad 1 \mathrm{x}-\Delta \mathrm{H}_f \mathrm{H}_2 \mathrm{O} \quad 2 \mathrm{x} \Delta \mathrm{H}_f \mathrm{HNO}_3 \quad 1 \mathrm{x} \Delta \mathrm{H}_f \mathrm{NO}$
M2 correct signs associated with the appropriate $\Delta H_f$ values/terms used for the calculation of $\Delta \mathrm{H}_{\text {reaction }}$ M3 $\Delta \mathrm{H}_{\text {reaction }}=-(102-286)+(-346+91.1)=-70.9 \mathrm{~kJ} \mathrm{~mol}^{-1}$
(c) M1 nitrogen has a triple bond
M2 EITHER
high energy is needed to break the bond
OR
at normal temperatures there is not enough energy to break the bond / to overcome the activation energy
(d) lightning
(e)(i) M1 define homogeneous
(homogeneous catalyst is) in the same phase / state as the reactants
M2 and M3 Define catalyst
All 3 points scores 2 marks. Any 2 points scores 1 mark
increase the rate
AND
lowers the activation energy
AND
without being chemically altered at the end of the reaction / are regenerated at the end of the reaction
(e)(ii)$\begin{aligned} & \text { M1 NO } \mathrm{N}_2+\mathrm{SO}_2 \rightarrow \mathrm{NO}+\mathrm{SO}_3 \\ & \text { M2 NO }+1 / 2 \mathrm{O}_2 \rightarrow \mathrm{NO}_2\end{aligned}$
Question:
The Group 17 elements, chlorine, bromine and iodine, are non-metals that show trends in their physical and chemical properties.
(a) Describe the trend in the colour of the Group 17 elements down the group.[1]
(b) The Group 17 elements can oxidise many metals to form halides.
(i) Describe the relative reactivity of the elements in Group 17 as oxidising agents.[1]
(ii) Chlorine reacts with hot tin metal to form tin(IV) chloride, $\mathrm{SnCl}_4$.
$\mathrm{SnCl}_4$ is a colourless liquid at room temperature that reacts vigorously with water to form an acidic solution.
Suggest the type of structure and bonding shown by $\mathrm{SnCl}_4$. Explain your answer.[2]
(c) The Group 17 elements form soluble halides with sodium.
(i) Describe what is seen when dilute $\mathrm{AgNO}_3(\mathrm{aq})$ is added to $\mathrm{NaBr}(\mathrm{aq})$ followed by aqueous ammonia. [2]
(ii) $\mathrm{NaCl}$ reacts with concentrated $\mathrm{H}_2 \mathrm{SO}_4$ to form $\mathrm{HCl}$ and $\mathrm{NaHSO}_4$.
Explain the difference between the reactions of concentrated $\mathrm{H}_2 \mathrm{SO}_4$ with $\mathrm{NaCl}$ and with NaI. Your answer should refer to the role of the sulfuric acid in each reaction.[3]
(d) The hydrogen halides are useful reagents in organic and inorganic reactions.
(i) Describe and explain the trend in the boiling points of the hydrogen halides, $\mathrm{HCl}, \mathrm{HBr}$ and HI.[2]
(ii) Describe and explain the trend in the thermal stabilities of the hydrogen halides, $\mathrm{HCl}, \mathrm{HBr}$ and $\mathrm{HI}$.
(e) Lucas’s reagent is a mixture of $\mathrm{HCl}$ and $\mathrm{ZnCl}_2$. Primary, secondary and tertiary alcohols can be distinguished by their reaction with Lucas’s reagent.
Alcohols react with the $\mathrm{HC} l$ in Lucas’s reagent to form halogenoalkanes.
$\mathrm{ZnCl}_2$ acts as a homogeneous catalyst for these reactions.
(i) Explain the meaning of the term homogeneous. [1]
(ii) Pentan-3-ol, $\mathrm{C}_2 \mathrm{H}_5 \mathrm{CH}(\mathrm{OH}) \mathrm{C}_2 \mathrm{H}_5$, reacts slowly with $\mathrm{HCl}$ to form a secondary halogenoalkane.
Complete the equation for this reaction using structural formulae.
$\mathrm{C}_2 \mathrm{H}_5 \mathrm{CH}(\mathrm{OH}) \mathrm{C}_2 \mathrm{H}_5+$
(iii) The fastest reaction shown by Lucas’s reagent is with a tertiary alcohol.
Draw the structure of the tertiary alcohol that is an isomer of pentan-3-ol.[1]
(iv) Tertiary alcohols tend to react with Lucas’s reagent using the same mechanism as in their reaction with $\mathrm{HCl}$.
Suggest the type of reaction shown by tertiary alcohols with Lucas’s reagent.[1][Total: 17]
▶️Answer/Explanation
Ans:
(a) darker / stronger / deeper down the group 1
(b)(i) weaker oxidising agents / (relative reactivity as oxidising agents) decreases down the group 1
(b)(ii) M1 (structure =) simple / molecular, because it has a low melting / boiling point
M2 (bonding =) covalent, because it is hydrolysed
(c)(i) M1 cream ppt / solid
M2 (ppt / solid) partially dissolves in (aqueous) ammonia
(c)(ii) M1 Acid behaviour of $\mathrm{H}_2 \mathrm{SO}_4$ $\mathrm{H}_2 \mathrm{SO}_4$ acts as an acid with $\mathrm{C} l^{-}$ $\mathrm{OR}$ acid / base reaction with $\mathrm{Cl}^{-}$
M2 Oxidising behaviour of $\mathrm{H}_2 \mathrm{SO}_4$ $\mathrm{H}_2 \mathrm{SO}_4$ acts as an oxidising agent with $\mathrm{I}^{-}$ $\mathrm{OR} \mathrm{H}_2 \mathrm{SO}_4$ does not oxidise $\mathrm{Cl}^{-}$
M3
Products formed (for iodide reaction) $\mathrm{I}_2 / \mathrm{S} / \mathrm{SO}_2 / \mathrm{H}_2 \mathrm{~S}$ is formed $\mathrm{OR}$ (for chloride reaction) (only) $\mathrm{HC} l$ is formed OR
Comparison of oxidising strength $\mathrm{H}_2 \mathrm{SO}_4$ not strong enough to / cannot oxidise $\mathrm{Cl}^{-}$(to $\mathrm{Cl}_2$ ) $\mathrm{OR} \mathrm{I}^{-}$more powerful reducing agent than $\mathrm{Cl}^{-}$
(d)(i) M1 increases (down the group) because of increasing VdW
M2 because of increasing number of electrons
(d)(ii) M1 less stable (down the group) / decreases
M2 lower H–Hal bond enthalpy / energy
(e)(i) in the same phase / state
(e)(ii) $\mathrm{C}_2 \mathrm{H}_5 \mathrm{CH}(\mathrm{OH}) \mathrm{C}_2 \mathrm{H}_5+\mathrm{HCl} \rightarrow \mathrm{C}_2 \mathrm{H}_5 \mathrm{CH}(\mathrm{Cl}) \mathrm{C}_2 \mathrm{H}_5+\mathrm{H}_2 \mathrm{O}$
(e)(iii)
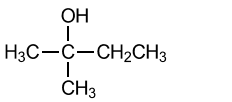
(e)(iv) substitution