Question:
Which statements are correct?
1 Aluminium chloride dissolves in water to give an acidic solution.
2 Magnesium chloride dissolves in water to give a solution of pH close to 7.
3 Sodium chloride dissolves in water to give an alkaline solution.

▶️Answer/Explanation
Ans:B
Question
When dealing with a spillage of metallic sodium it is important that no toxic or flammable products are formed.
Which material should be used if there is a spillage of metallic sodium?
A dilute hydrochloric acid
B ethanol
C sand
D water spray
▶️Answer/Explanation
Ans:C
Question
The elements in Period 3 and their compounds show trends across the period from sodium to chlorine.
Which row is correct?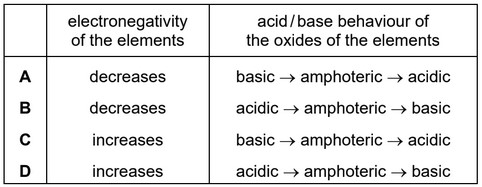
Answer/Explanation
Ans: C
Question
A student investigated the chloride of a Period 3 element. This is what the student wrote down as a record.
The compound was a white crystalline solid. It dissolved easily in water to give a solution of pH 12. When placed in a test-tube and heated in a roaring Bunsen flame, the compound melted after several minutes heating.
What can be deduced from this record?
A At least one of the recorded observations is incorrect.
B The compound was magnesium chloride, \(MgCl_2\).
C The compound was phosphorus pentachloride, \(PCl_5\).
D The compound was sodium chloride, NaCl.
Answer/Explanation
Ans: A
Question
A student reacts 0.100 mol of each of sodium, magnesium and phosphorus atoms separately with an excess of oxygen.
Which rows are correct?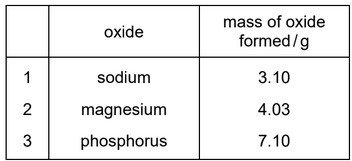
A 1, 2 and 3 B 1 and 2 only C 1 and 3 only D 2 and 3 only
Answer/Explanation
Ans: A
Question
Element X is in Period 3. Element X forms a solid oxide Y.
Y reacts with hot concentrated hydrochloric acid. Y reacts with hot aqueous sodium hydroxide to
form a compound in which X is part of an anion.
How many p electrons does one atom of X have in its outer shell?
A 0 B 1 C 2 D 3
Answer/Explanation
Answer: B
Question
R is an oxide of Period 3 element T. 5.00 g of R contains 2.50 g of T.
What is T?
A magnesium
B aluminium
C silicon
D sulfur
Answer/Explanation
Answer: D
Question
An element, Y, reacts according to the following sequence.
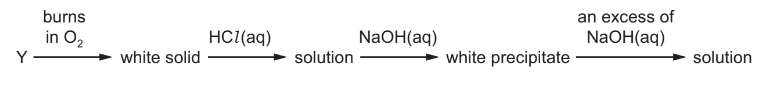
What could be element Y?
A Na B Mg C Al D P
Answer/Explanation
Answer C
Question
Which element requires the least number of moles of oxygen for the complete combustion of
1 mol of its atoms?
A aluminium
B magnesium
C phosphorus
D sodium
Answer/Explanation
Answer D
Question
A solid Period 3 element, Q, is reacted with oxygen gas. Compound R is formed. When R is added to water the pH decreases.
What could be the empirical formula of R?
A Q 2O4 B Q2O5 C Q4O10 D Q5O2
Answer/Explanation
Answer:
B
Question
X, Y and Z are elements in Period 3 of the Periodic Table. The results of some experiments carried out with compounds of these elements are shown.
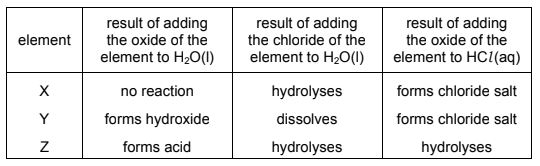
Which statement could be correct?
A X is Al and Y is Mg.
B X is Si and Y is Na.
C Y is Al and Z is P.
D Y is Na and Z is Al .
Answer/Explanation
Answer:
A
Question
Which oxide will cause an increase in pH when added to water?
A MgO B Al2O3 C SiO2 D SO2
Answer/Explanation
Answer A
Question
Sodium burns in oxygen giving out heat energy and forming the compound Na$_2$O. The equation for this reaction is shown.
\(2Na(s) + \frac{1}{2}O_{2}(g) \rightarrow Na_{2}O(s)\)
Which statement about the reaction is correct?
A ∆Hθ for the reaction is equal to twice the bond energy of the Na–O bond.
B ∆Hθ for the reaction is positive.
C The equation represents the standard enthalpy change of combustion of sodium.
D The equation represents the standard enthalpy change of formation of sodium oxide.
Answer/Explanation
Answer D
Question
Which statement explains the observation that magnesium hydroxide dissolves in aqueous ammonium chloride, but not in aqueous sodium chloride?
A The ionic radius of the NH4+ ion is similar to that of Mg2+ but not that of Na+.
B NH4Cl dissociates less fully than NaCl.
C The Na+ and Mg2+ ions have the same number of electrons.
D The NH4+ ion can donate a proton.
Answer/Explanation
Answer D
Question
X and Y are elements in Period 3 of the Periodic Table.
● The oxide of X is a solid at room temperature. This oxide has a giant structure.
● The chloride of X does not react with water.
● Argon is the only element in Period 3 with a lower melting point than Y.
What could be the formula of a compound formed between elements X and Y?
A Al2S3 B MgS C NaCl D PCl5
Answer/Explanation
Answer C
Question
Sodium and sulfur are burned separately in oxygen.
Each reaction has a distinctive coloured flame.
Which row is correct?
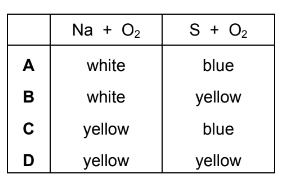
Answer/Explanation
Answer C
Question
X, Y and Z are three elements in the third period.
● X reacts with chlorine to give a liquid product.
● Y reacts with chlorine to give a solid product that dissolves in water to give a solution of pH 7.
● Z reacts with chlorine to give a solid product that dissolves in water to give a solution of pH 6.
Which elements are good conductors of electricity?
A X and Y B Y and Z C Y only D Z only
Answer/Explanation
Answer:
B
Question
Compound T is a white crystalline solid.
When a sample of T was mixed with aqueous sodium hydroxide and heated, a pungent smelling gas was produced which turned damp red litmus paper blue. This same gas produced dense white smoke with hydrogen chloride gas.
Further testing of a solution of T with barium chloride solution produced a dense white precipitate which did not dissolve when dilute hydrochloric acid was added to the mixture.
What is the identity of compound T?
A ammonium carbonate
B ammonium sulfate
C sodium carbonate
D sodium sulfate
Answer/Explanation
Answer: B
Question
Aluminium is extracted from aluminium oxide by electrolysis.
Which statements are correct?
1 Aluminium oxide has an extremely high melting point.
2 Bauxite is added to the aluminium oxide to lower its melting point.
3 Oxygen produced at the graphite cathode reacts with the graphite to produce \(CO_{2}\).
The responses A to D should be selected on the basis of
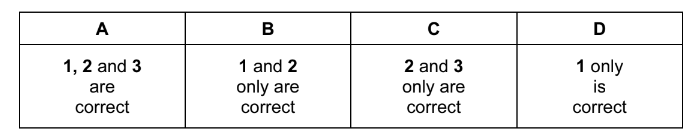
Answer/Explanation
Ans:D
Question
X is the oxide of a Period 3 element. X reacts with water to give an acidic solution.
A solution is prepared by reacting 0.100g of X with excess water. This solution was neutralised by exactly \( 25.0cm^{3}\) of \( 0.100moldm^{–3}\) sodium hydroxide solution. What could be the identity of X?
A \(Al _{2}O_{3} \) B MgO C \( P_{4}O_{10}\) D \(SO_{3}\)
Answer/Explanation
Ans:D
Question
Which statements about calcium oxide are correct?
1 It reacts with cold water.
2 It is produced when calcium nitrate is heated.
3 It can be reduced by heating with magnesium.
The responses A to D should be selected on the basis of
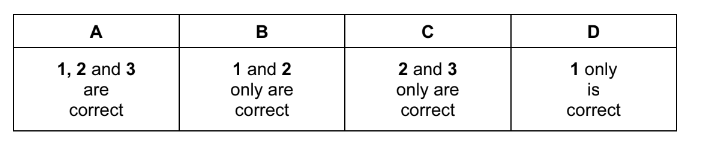
▶️Answer/Explanation
Ans:B
Question
Which oxide does not react with cold dilute sodium hydroxide to produce a salt?
A \( Al_{ 2}O_{3}\) B \(P_{4}O_{10}\) C \( SO_{2}\) D \( SiO_{2}\)
Answer/Explanation
Ans:D
Question
Use of the Data Booklet is relevant to this question.
Sir Humphrey Davy discovered boron, calcium, magnesium and sodium.
Which of these elements has the second smallest atomic radius in its group and the third lowest first ionisation energy in its period?
A boron
B calcium
C magnesium
D sodium
Answer/Explanation
Ans:C
Question
When calcium is burnt in oxygen, what colour is the flame?
A green
B red
C white
D yellow
Answer/Explanation
Ans:B
Question
At the age of 17, in a woodshed in Ohio, Charles Martin Hall discovered the commercial process
for the production of aluminium metal by the electrolysis of a mixture of bauxite, \(Al_2O_3\), and
cryolite, \(Na_3AlF_6\).
What is the main purpose of the cryolite?
A \(Al_2O_3\) is covalent, and \(AlF_6^{3–}\) ions interact with it to produce \(Al^{3+}\) ions which can be discharged at the cathode.
B Cryolite is a base, forming \(NaAlO_2\) with bauxite, enabling aluminium to be discharged at the anode.
C Cryolite minimises the release of \(O^{2–}\) ions at the graphite anodes, which are otherwise burnt away to CO.
D Cryolite reduces the melting point of the bauxite.
Answer/Explanation
Ans: D