Question
Boron forms many useful compounds.
(a) The compound diborane, \(B_{2}H_{6}\), can be used as a rocket fuel.
It can be prepared by the reaction of boron trifluoride, \(BF_{3}\), with sodium borohydride, \(NaBH_{4}\).
Balance this equation.
…….\(BF_{3}\) + …….NaBH_{4} → …….B_{2}H6 + …….\(NaBF_{4}\)
(b) Primary and secondary alcohols can be formed by the reaction of carbonyl compounds with
\(NaBH_{4}\), which is a source of hydride ions, \(H^{–}\)
. Complete the mechanism for the reaction of butanone with hydride ions, \(H^{–}\) , and draw the intermediate in the box. Include all necessary curly arrows and relevant dipoles.
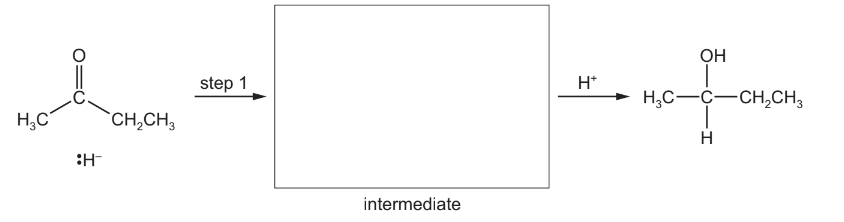
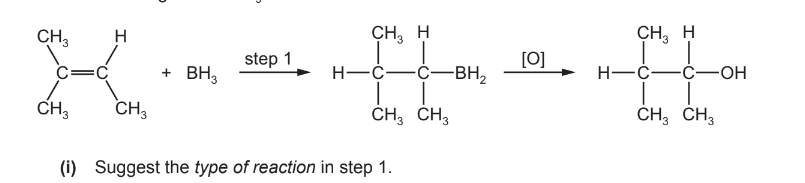
(c) Borane, \(BH_{3}\), is used to synthesise alcohols from alkenes. The reaction occurs in two steps. The \(BH_{2}\) group from \(BH_{3}\) bonds to the least substituted carbon atom of the double bond, and the remaining H from \(BH_{3}\) bonds to the other carbon.
(ii) The diol Y can be prepared by the same method.
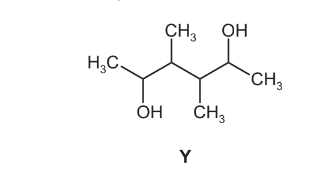
Draw the structure of the diene which could be used to prepare diol Y.
(d) Benzene,\( C_{6}H_{6}\), and borazine, \(B_{3}N_{3}H_{6}\), have planar, cyclic structures.
(i) Describe the structure of and bonding in benzene, \(C_{6}H_{6}\).
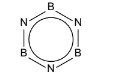
(ii) In borazine, \(B_{3}N_{3}H_{6}\), the boron and nitrogen atoms alternate around the ring. Each ring atom has a single hydrogen atom bonded to it.
All boron-nitrogen bonds in borazine are 0.144nm in length, whereas in simple compounds
B–N and B=N bond lengths are 0.154nm and 0.136nm respectively. Suggest and draw the structure of borazine.
Answer/Explanation
(a) \( 4BF_{3} + 3NaBH_{4}\) → \(2B_{2}H6 + 3NaBF_{4}\)
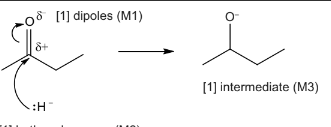
[1] both curly arrows (M2)
arrow must come from lone pair
(c) (i) (electrophilic) addition
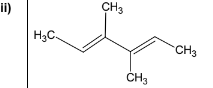
(d) (i) any four of
M1:
σ-bonds between C–C or C–H
M2: π-bonds formed from overlap of p-orbitals
M3: (π-bonds/electrons) above and below the ring
M4:bonds/electrons are delocalised
M5: bond angle 120°
M6: intermediate C–C bond length/all C–C same length/ strength
M7: carbons are \(sp^{2}\) hybridised
(ii) correct delocalised structure of borazine
Question
D, E, F, and $\mathbf{G}$ are four consecutive elements in the fourth period of the Periodic Table. (The letters are not the actual symbols of the elements.)
D is a soft, silvery metal with a melting point just above room temperature. Its amphoteric oxide, $\mathbf{D}_2 \mathrm{O}_3$, has a melting point of $1900^{\circ} \mathrm{C}$ and can be formed by heating $\mathbf{D}$ in oxygen.
$\mathbf{G}$ is a solid that can exist as several different allotropes, most of which contain $\mathbf{G}_8$ molecules. $\mathbf{G}$ burns in air to form $\mathbf{G O}_2$ which dissolves in water to form an acidic solution. This solution reacts with sodium hydroxide to form the salt $\mathrm{Na}_2 \mathrm{GO}_3$.
(a) Suggest the identities of $\mathbf{D}$ and $\mathbf{G}$.
D……………………………………………… G………………………………………[1]
(b) Write equations for the reactions of $\mathrm{D}_2 \mathrm{O}_3$ with
(i) hydrochloric acid,[2]
(ii) sodium hydroxide.[2]
(c) Suggest the type of bonding and structure in $\mathbf{D}_2 \mathrm{O}_3$.[1]
(d) Write an equation for the formation of an acidic solution when $\mathbf{G O}_2$ dissolves in water.[1][Total: 7]
▶️Answer/Explanation
Ans:
(a) D = Ga G = Se
(b) (i) $\begin{aligned} & \mathbf{D}_2 \mathrm{O}_3+6 \mathrm{HCl} \rightarrow 2 \mathbf{D C l} l_3+3 \mathrm{H}_2 \mathrm{O} \\ & \mathrm{M} 1=\text { species; } \\ & \mathrm{M} 2=\text { balancing }\end{aligned}$
(ii) $\begin{aligned} & \mathbf{D}_2 \mathrm{O}_3+2 \mathrm{NaOH}+7 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{NaD}(\mathrm{OH})_4\left(\mathrm{H}_2 \mathrm{O}\right)_2 \mathrm{OR} \\ & \mathbf{D}_2 \mathrm{O}_3+2 \mathrm{NaOH}+3 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{NaD}(\mathrm{OH})_4 \mathrm{OR} \\ & \mathbf{D}_2 \mathrm{O}_3+2 \mathrm{NaOH}_{\rightarrow 2 \mathrm{NaDO}}^2+\mathrm{H}_2 \mathrm{O} \mathrm{OR} \\ & \mathbf{D}_2 \mathrm{O}_3+2 \mathrm{OH}^{-}+7 \mathrm{H}_2 \mathrm{O} \rightarrow 2\left[\mathrm{D}(\mathrm{OH})_4\left(\mathrm{H}_2 \mathrm{O}\right)_2\right]^{-} \mathrm{OR} \\ & \mathbf{D}_2 \mathrm{O}_3+2 \mathrm{OH}^{-}+3 \mathrm{H}_2 \mathrm{O} \rightarrow 2\left[\mathrm{D}(\mathrm{OH})_4\right]^{-} \mathrm{OR} \\ & \mathbf{D}_2 \mathrm{O}_3+2 \mathrm{OH}^{-} \rightarrow 2 \mathrm{DO}_2^{-}+\mathrm{H} 2 \mathrm{O} \\ & \\ & \mathrm{M} 1=\text { species; } \\ & \mathrm{M} 2=\text { balancing }\end{aligned}$
(c) giant ionic / ionic lattice
(d) $\quad \mathbf{G O}_2+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{H}_2 \mathbf{G O}_3$