Question
When considering one molecule of ethene, which row describes both the hybridisation of the atomic orbitals in the carbon atoms and the overall bonding
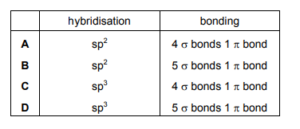
▶️Answer/Explanation
Ans:B
Question:
The diagram shows the structure of compound Q.
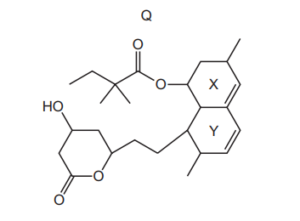
Two of the rings, X and Y, contain a C=C bond. Which row is correct?
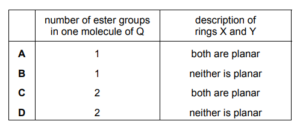
▶️Answer/Explanation
Ans:D
Question
Structural isomerism and stereoisomerism should be considered when answering this question.
How many isomers with the formula $\mathrm{C}_4 \mathrm{H}_8$ have structures that contain a $\pi$ bond?
A 1
B 2
C 3
D 4
▶️Answer/Explanation
Ans:D
Question
Which row of the table is correct?
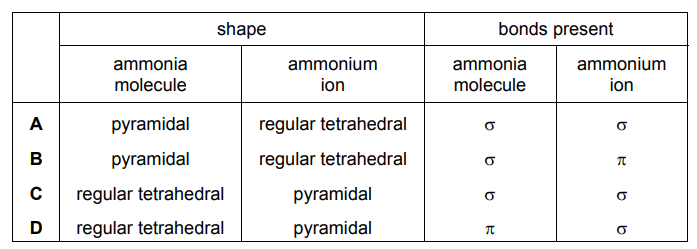
▶️Answer/Explanation
Ans:A
Question
People who take statin drugs to control their blood cholesterol may also take ‘coenzyme $Q_{10}$ ‘. The diagram shows a simplified structure of one form of this coenzyme.
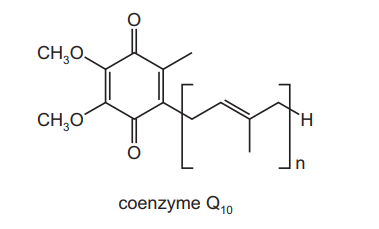
Which row describes this structure correctly?
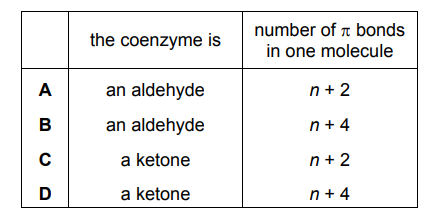
▶️Answer/Explanation
Ans:D
Question
Which row is correct?
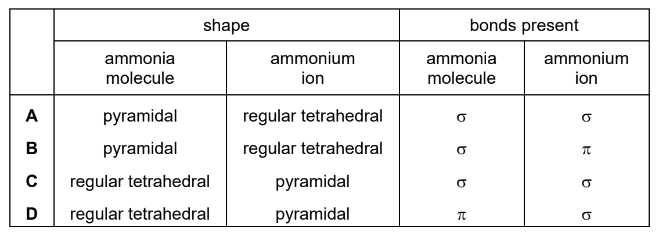
Answer/Explanation
Answer A
Question
The structure of tiglic acid is CH3CH=C(CH3)CO2H.
Which statements about the properties of one molecule of this acid are correct?
1 It contains two π bonds.
2 It contains four lone pairs of electrons.
3 It has all its atoms in the same plane.
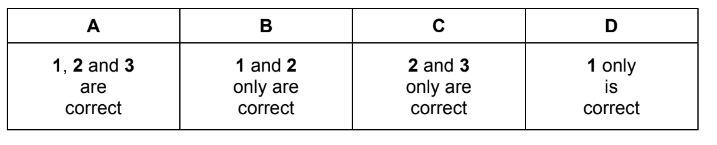
▶️Answer/Explanation
Answer B
Question
Four compounds are shown.
C2H4 C2H5OH CH3CHO CH3CO2H
How many of these compounds have an odd number of σ bonds?
A 1 B 2 C 3 D 4
Answer/Explanation
Answer B
Question
The diagrams show two different compounds.
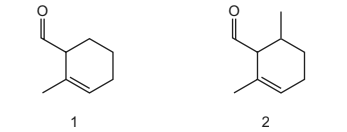
What is
● the total number of structural isomers, including compound 2, that could be formed
by adding a second methyl group to the ring of compound 1,
● the number of π electrons in each compound?
Answer/Explanation
Answer D
Question
Which elements can form π bonds in their compounds?
1 carbon
2 oxygen
3 nitrogen
Answer/Explanation
Answer: A
Question
The diagrams show two different compounds.
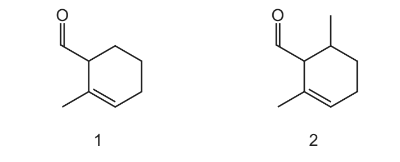
What is
-
-
- the total number of structural isomers, including compound 2, that could be formed by adding a second methyl group to the ring of compound 1,
- the number of π electrons in each compound?
-
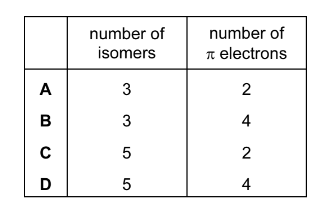
Answer/Explanation
Answer: D
Question
The double bond between the two carbon atoms in an ethene molecule consists of one σ bond and one π bond.
Which orbitals overlap to form each of these bonds?
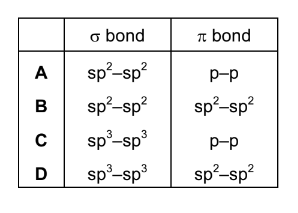
Answer/Explanation
Ans:A
Question
Carvone is found in spearmint.
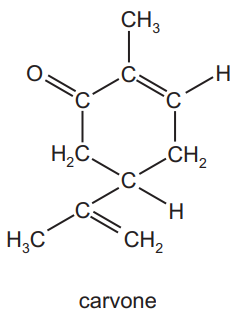
How many σ and π bonds are present in this molecule?
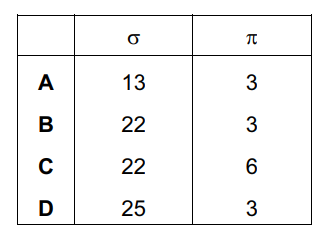
Answer/Explanation
Ans:
D
Question
What is always involved in a carbon-carbon π bond?
- a shared pair of electrons
- a sideways overlap of p orbitals
- delocalised electrons
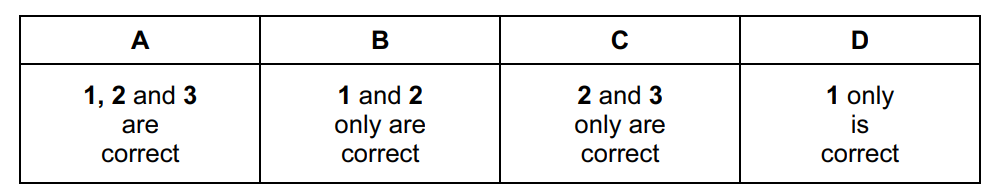
Answer/Explanation
Ans:
B