Question
Over one million tonnes of hydrogen cyanide, $\mathrm{HCN}$, are produced each year using the Andrussow process. The overall equation for the reaction is shown.
$
\mathrm{CH}_4(\mathrm{~g})+\mathrm{NH}_3(\mathrm{~g})+1 \frac{1}{2} \mathrm{O}_2(\mathrm{~g}) \rightleftharpoons \mathrm{HCN}(\mathrm{g})+3 \mathrm{H}_2 \mathrm{O}(\mathrm{g})
$
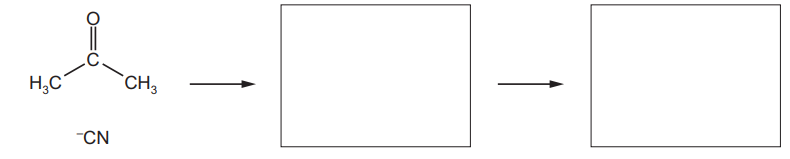
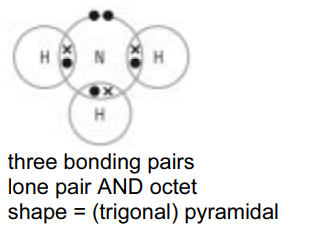
(a) (i) Draw a dot-and-cross diagram to represent the bonding in a molecule of ammonia, $\mathrm{NH}_3$, and state the shape of the molecule.
shape of molecule[3]
(ii) A molecule of hydrogen cyanide, $\mathrm{HCN}$, is shown.
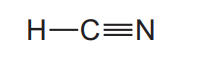
The bonding between the carbon and nitrogen atoms consists of one sigma (σ) bond andtwo pi (π) bonds.
Sketch the shape of the sigma bond and one of the pi bonds in the space below.
Show clearly the position of the atomic nuclei in each diagram.
(b) The reaction exists as a dynamic equilibrium.
(i) Explain what is meant by the term dynamic equilibrium……………………………………………………………………………………………………………………………. [1]
(ii) State and explain how the amounts of the chemicals present in the equilibrium mixture will
change when the pressure is increased………………………………………………………………………………………………………………………. [2]
(c) The process uses a platinum catalyst, which increases the rate of reaction.
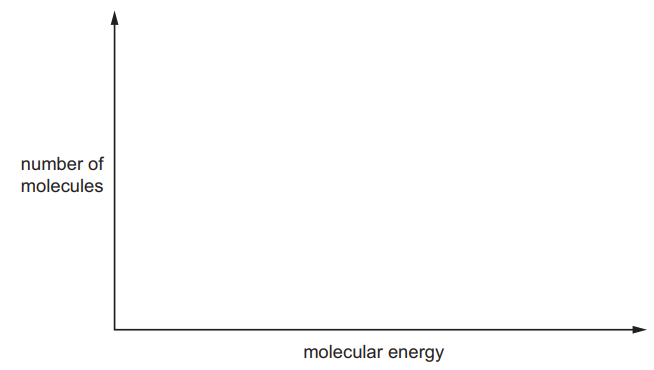
Sketch a Boltzmann distribution on the axes given below and use your diagram to explain how the platinum catalyst increases the rate of the reaction.
 [3]
[3]
(d) The reaction of hydrogen cyanide with propanone is an important first step in many organic syntheses.
(i) Give the full name of the mechanism of this reaction.
……………………………………………………………………………………………………………………… [1]
(ii) Complete the diagram to show the mechanism of the reaction of hydrogen cyanide with propanone.
Draw the structure of the intermediate and the product of the reaction. Include all relevant charges, partial charges, curly arrows and lone pairs.
 [5][Total: 17]
[5][Total: 17]
▶️Answer/Explanation
Ans:
(a) (i)
(ii)
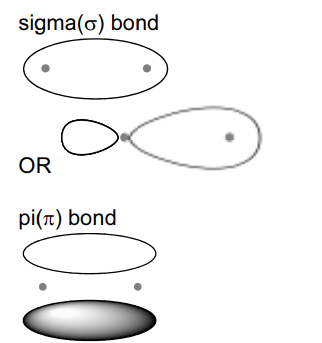
(b) (i) forward and backward reactions occurring at same rate OR the rate of forward and backward reactions are equal
(ii) $\mathrm{M} 1=$ decreased yield of products $/$ less products formed $/$ ora M2 = left-hand side has fewer moles of gas OR equilibrium shifts to the left
(c)
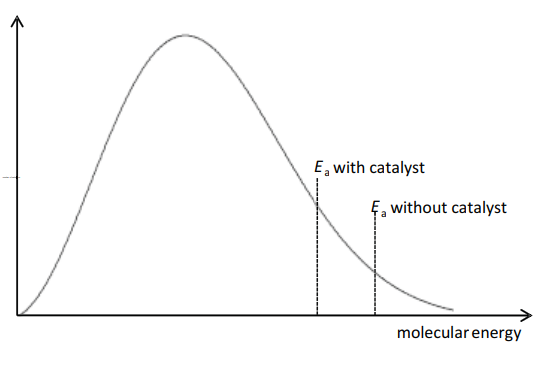
M1 = correct Boltzmann curve
M2, M3 any 2 from:
- line for both $E_a$ values or statement in text that catalyst lowers $E_a$
- (catalyst) increases proportion/number of molecules/particles with energy $\geqslant$ activation energy
- so more frequent successful collisions
(d) (i) nucleophilic addition
(ii)
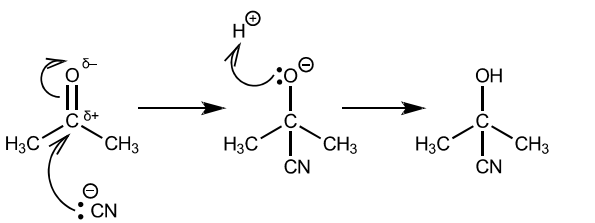
correct dipole on carbonyl
curly arrow from lone pair on $\mathrm{CN}^{-} \mathrm{AND}$ from $\mathrm{C}=\mathrm{O}$ to $\mathrm{O}$ correct intermediate curly arrow from lone pair on $\mathrm{O}^{-}$to $\mathrm{H}^{+}$ correct product
Question
Nitrogen molecules, \(N_2(g)\), contain two atoms attracted to each other by a triple covalent bond.
(a) Describe how the triple covalent bond forms in a \(N_2(g)\) molecule. Refer to orbital overlap and hybridisation in your answer.
(b) Nitrogen oxides, \(NO_2\) and NO, are produced in internal combustion engines. Release of these gases into the atmosphere leads to the formation of photochemical smog.
(i) Outline how nitrogen oxides are involved in the formation of photochemical smog.
(ii) Construct an equation to demonstrate how a catalytic converter reduces the amount of nitrogen oxide gases released into the atmosphere.
(c) \(N_2(g)\) is very unreactive. It is difficult to make ammonia, \(NH_3(g)\), directly from its elements but it can be made from \(NH_4Cl(s)\). Identify a reagent and the conditions required to make \(NH_3(g)\) from \(NH_4Cl(s)\).
(d) \(25cm^3\) of 0.10\(moldm^{–3}\) HCl(aq) is added to a beaker and its pH is recorded. \(50cm^3\) of 0.10\(moldm^{–3}\) \(NH_3(aq)\) is added to the HCl(aq) in 5\(cm^3\) portions.
The pH of the mixture is monitored until all the \(NH_3(aq)\) is added. HCl is a strong Brønsted-Lowry acid.
(i) Describe what is meant by a strong Brønsted-Lowry acid.
(ii) \(NH_3\) is a weak base.
Construct an equation that shows the behaviour of \(NH_3\) as a weak Brønsted-Lowry base when dissolved in water.
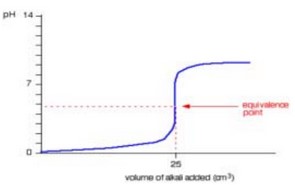
(iii) On Fig. 2.1 sketch a graph to show the change in pH which occurs when HCl(aq) is titrated with \(NH_3(aq)\) as described in (d).
Answer/Explanation
Answer:
(a) M1 one sigma / \(\sigma \) bond and two pi / π bonds
M2 sp hybridisation (in each N atom)
M3 sigma / \(\sigma \) forms from direct / head-on / end-on overlap of orbitals
AND pi / \(\pi \) forms sideways / lateral overlap of (p) orbitals
(b) (i) M1 react with (unburnt) hydrocarbons
M2 (form) PAN / peroxyac(et)yl nitrate
(ii) \(2NO + 2CO \rightarrow 2CO_2 + N2\) OR \(NO_2 + 2CO \rightarrow 1⁄2N_2 + 2CO_2\)
(c) any Group 1 hydroxide or \(Ca(OH)_2\) / \(Sr(OH)_2 / Ba(OH)_2\)
(d) (i) M1 proton / \(H^+\) donor
M2 fully dissociates (in aqueous solution / water / solvent)
(ii) \(NH_3 + H_2O ⇌ NH_4^+ + OH^–\)
(iii) M1 correct basic shape extending to ~50\(cm^3\) with vertical portion of curve at 25 \(cm^3\)
M2 initial pH at 0–2 (based on idea that HCl is a strong acid) AND final pH at between 8–12 (based on idea that \(NH_3\) is a
weak alkali)