Haloalkanes and Haloarenes : Notes and Study Materials -pdf
Notes and Study Materials
- Concepts of Haloalkanes and Haloarenes
- Master File Haloalkanes and Haloarenes
- NCERT Solutions for – Haloalkanes and Haloarenes
- NCERT Exemplar Solutions for – Haloalkanes and Haloarenes
- Mind Map of Haloalkanes and Haloarenes
- Concept Map of Haloalkanes and Haloarenes
- Past Many 12th Board Years of Haloalkanes and Haloarenes
Examples and Exercise
CBSE Class 12th Chemistry Notes: Haloalkanes and Haloarenes
Get important revision notes on CBSE Class 12th Chemistry, Chapter 10 – Haloalkanes and Haloarenes. These notes are based on latest CBSE syllabus and are helpful for quick revision of the chapter. CBSE Class 12th Chemistry: Chapter 10 – The Haloalkanes and Haloarenes, to give you a quick glance of the chapter. These NCERT based notes are very important for CBSE Class 12th Chemistry.
The main topics covered in this part are:
• Introduction to haloalkanes and haloarenes
• Classification of haloalkanes
• Nature of C─X bond in haloalkanes
• Preparation of haloalkanes
• Physical properties of haloderivatives
• Reactions of Haloalkanes
• Stereoisomerism
• Definition: Enantiomers, Racemic mixture, Racemisation
The key notes of the chapter are as follows:
Haloalkane and Haloarene:
In aliphatic or aromatic compounds, the replacement of hydrogen atom(s) by halogen atom(s) results in the formation of alkyl halide (haloalkane) and aryl halide (haloarene), respectively.
In case of haloalkanes, halogen atom is attached to the sp3 hybridised carbon atom of an alkyl group whereas in haloarenes, halogen atom is attached to sp2 hybridised carbon atom of an aryl group.
Classification of Haloalkanes:
The halogen derivatives of hydrocarbons may be classified as fluoro, chloro, bromo or iodo compounds according to the type of halogen present.
Depending upon the number of halogens present, the halogen derivatives can be classified as mono, di, tri or polyhalo compounds.
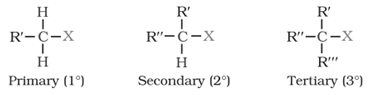
On the basis of the nature of the carbon to which halogen atom is attached, halogen derivatives are classified as 1o, 2o, 3o, allylic, benzylic, vinylic and aryl derivatives.
For example:
1o, 2o and 3o halides: halogen atom is bonded to primary, secondary or tertiary carbon atom of an an alkyl group.
Allylic halides: Halogen atom is bonded to an sp3-hybridised carbon atom next to carbon-carbon double bond (C=C) i.e. to an allylic carbon.
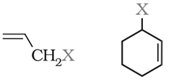
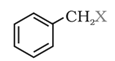
Benzylic halides: Halogen atom is attached to an sp3 – hybridised carbon atom next to an aromatic ring.
Vinylic halides: Halogen atom is bonded to an sp2-hybridised carbon atom of a carbon-carbon double bond (C = C).
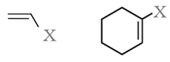
Aryl halides: Halogen atom is bonded to the sp2-hybridised carbon atom of an aromatic ring.
Note: Here X represents a halogen atom, i.e., X= F, Cl, Br, I.
Nature of C-X bond in haloalkanes
X is more electronegative than carbon. So, the C-X bond is polarized with C having a partial positive charge and X having a partial negative charge.
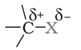
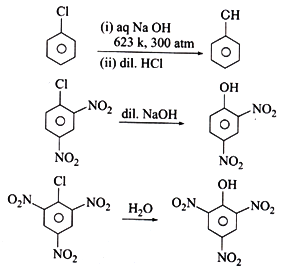
Preparation of Haloalkanes
Haloalkanes can be prepared by a number of methods:
1. By free radical halogenation of alkanes:
Chlorination or bromination of alkane usually gives a complete mixture of isomeric mono and poly halo alkanes.
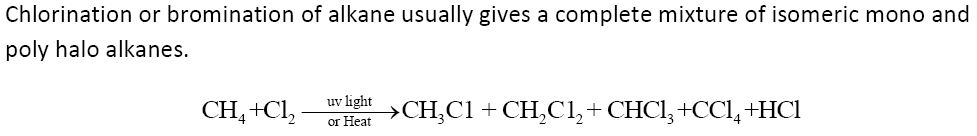
2. By electrophilic addition of HX to alkene:
An alkene is converted to corresponding alkyl halide by reaction with hydrogen chloride, hydrogen bromide or hydrogen iodide.
![]()
3. From alcohol:
The hydroxyl group of an alcohol is replaced by halogen on reaction with concentrated halogen acids, phosphorus halides or thionyl chloride to give the corresponding alkyl halide.
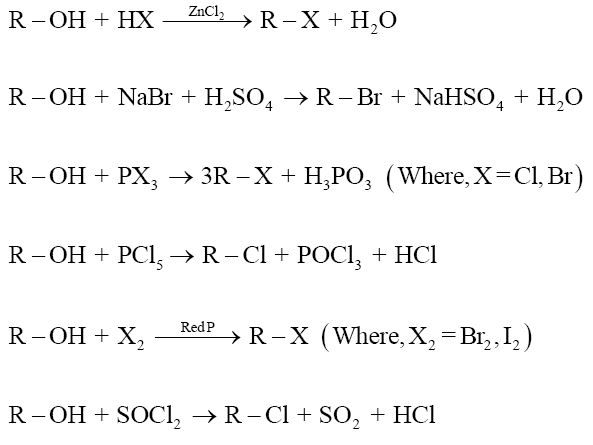
4. By halogen exchange:
(a) Finkelstein reaction:
Alkyl iodides can be prepared by the reaction of alkyl chlorides/ bromides with NaI in dry acetone.
![]()
(b) Swarts reaction:
Alkyl fluorides can be prepared by heating an alkyl chloride/bromide in the presence of a metallic fluoride such as AgF, Hg2F2, CoF2 or SbF3.
![]()
Preparation of haloarenes
Haloarenes can be synthesised by any of the following reactions:
1. By electrophilic substitution reaction:
This involves the direct halogenation of benzene ring in the presence of Lewis acid catalysts like iron or iron (III) chloride.
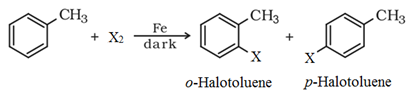
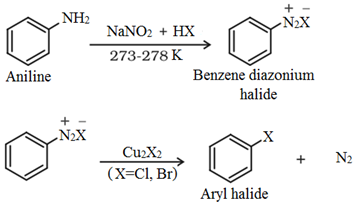
2. By Sandmeyer’s reaction:
Aniline is treated with sodium nitrite to give a diazonium salt which is then treated with cuprous chloride or cuprous bromide to produce the corresponding aryl halide:
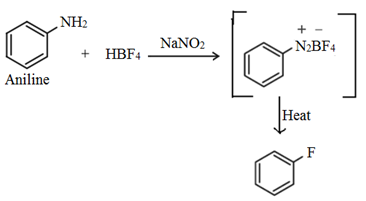
3. By Balz – Schiemann reaction:
This involves the conversion of aryl amines to aryl fluorides via diazotisation and subsequent thermal decomposition of the derived aromatic fluoborate to produce the corresponding aryl fluoride.
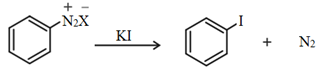
4. From diazonium group:
Treatment of diazonium salt with potassium iodide gives aryl iodide.
Physical properties of Haloderivatives
Physical properties of haloderivatives are different than those of the simple hydrocarbons. These are described below:
• Alkyl halides are colourless when pure but bromides and particularly iodides develop colour when exposed to light.
• The alkyl halides have higher molecular mass as compared to alkanes.
• Halogen compounds have higher boiling points than the corresponding hydrocarbon. This is because the greater polarity as well as higher molecular mass as compared to the parent hydrocarbon causes the intermolecular forces of attraction (dipole-dipole and van der Waals) to be stronger in the halogen derivatives.
• For monohalogen compounds, the boiling point increases with increasing molecular mass of the halogen group with a fixed hydrocarbon group,
• All halogen derivatives of hydrocarbon are insoluble in water as they are incapable of forming hydrogen bonds with water but alkyl halides are soluble in non-polar solvents, R‒F < R‒Cl < R‒Br < R‒I
• The density increases with increasing number and the atomic mass of the halogen.
• Halogen compounds are less inflammable than the hydrocarbons. The inflammability decreases with increasing halogen content.
Reactions of Haloalkanes
The reactions of haloalkanes may be divided into the three main categories:
(i) Nucleophilic substitution
(ii) Elimination reactions
(iii) Reaction with metals
Nucleophilic substitution: A nucleophile attacks the haloalkane which is having a partial positive charge on the carbon atom bonded to halogen. Halide ion separates following a substitution reaction.

Reactivity of Haloalkanes towards nucleophilic substitution:
For the same alkyl group, as we move from F to I, strength of C−X bond decreases, therefore, the reactivity order is: R− I > R−Br > R−Cl > R−F
Mechanism of nucleophilic substitution reaction:
The nucleophilic substitution reaction can proceed via SN1 mechanism or SN2mechanism.
• Unimolecular nucleophilic substitution, SN1: This type of nucleophilic substitution takes place in two steps, first step being the rate determining step involves the formation of carbonium ion.
The reactivity order of haloalkanes towards SN1reaction is:
1° R−X < 2° R−X < 3° R−X
• Bimolecular nucleophilic substitution, SN2: This type of nucleophilic substitution takes place in one step. The incoming nucleophile interacts with alkyl halide causing the C−X bond bond to break while forming a new C−OH bond.
The reactivity of alkyl halide towards SN2 reaction is:
3° R−X < 2° R−X < 1° R−X
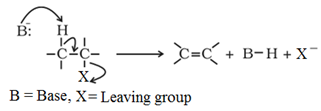
Elimination reactions:
When a haloalkane with β-hydrogen atom is heated with alcoholic solution of potassium hydroxide, there is elimination of hydrogen atom from β-carbon and a halogen atom from the α-carbon atom resulting in the formation of an alkene. The reaction follows the Saytzeff rule which states that “In dehydrohalogenation reactions, the preferred product is that alkene which has the greater number of alkyl groups attached to the doubly bonded carbon atoms.”
Reaction with metals:
Reaction with Magnesium: Alkyl halides react with magnesium in the presence of dry ether to form corresponding alkyl magnesium halide which is also known as Grignard’s reagent.
![]()
Recation with sodium: Alkyl halides react with sodium to form an alkane with double number of carbon atom than that present in alkyl halide. This reaction is also known as Wurtz reaction.
2R‒X + 2 Na → R ‒ R + 2NaX
Stereoisomerism
Stereoisomerism is due to the different orientation of atoms or groups in space. There are two types of stereoisomerism:
(i) Geometrical isomerism: It arises due to the presence of like groups on the same side of the plane (cis) or on the opposite side of the plane (trans).
(ii) Optical isomerism: It arises due to the presence of non-superimposable mirror images. Conditions for optical isomerism to take place are:
• Presence of chiral carbon or asymmetric carbon, i.e., The C attached to four different groups. Chiral carbon is denoted as C*.
• Presence of non-superimposable mirror images
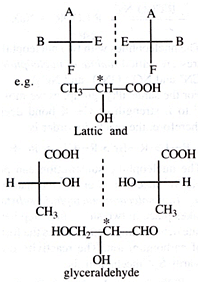
Enantiomers
The two non-superimposable mirror images are called enantiomers. Enantiomers have similar physical & chemical properties but differ in their
• effect on the plane polarised light
• reactivity towards chiral reagent.
Enantiomers are of two types:
• Dextrorotatory (+): These rotate the plane polarised light in a clockwise direction
• Laevorotatory (–): These rotate the plane polarised light in an anticlockwise direction
Racemic mixture
A mixture containing two enantiomers in equal proportions is called racemic mixture. A recemic mixture is optically inactive as the effect of one isomer gets cancelled by another isomer.
Racemisation
The process of conversion of enantiomers into a racemic mixture is known as racemisation.
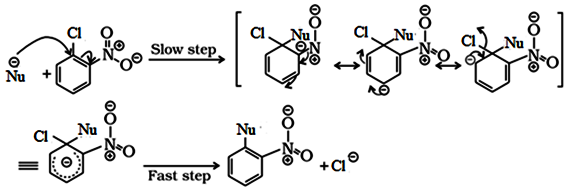
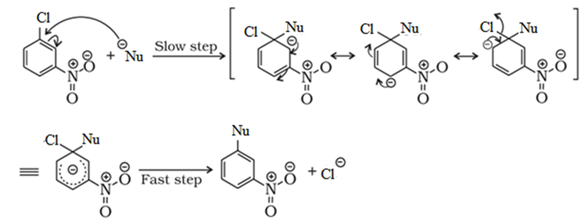
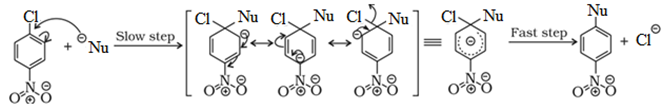
Reactions of Haloarenes
Nucleophilic substitution:
Aryl halides are almost unreactive towards nucleophilic substitution reaction. This is because of double character of C – X bond due to resonance. Therefore, it is difficult to remove X from C – X bond.
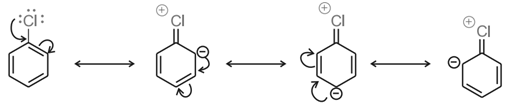
Effect of NO2 group on the reactivity of aryl halide towards nucleophilic substitution reactions:
Presence of an electron withdrawing group like NO2 group increases the reactivity of aryl halides towards nucleophilic substitution reaction.
NO2 group increases the reactivity more when present at o– and p– position due to the increased delocalization of negative charge involving NO2 group.
In Part-I you got acquainted with the haloalkane and haloarenes, their preparation, their physical and chemical properties, stereoisomerism, etc. In Part-II, you will get to know various important polyhalogeno compounds along with their preparation, properties and uses. These quick notes are prepared strictly according to the latest CBSE syllabus for Class 12th Chemistry.
The main topics covered in this part are:
o Polyhalogen compounds
• Dichloromethane, CH2Cl2
• Trichloromethane, CHCl3
• Triiodomethane, CHI3
• Tetrachloromethane, CCl4
• Freons
• Benzene hexachloride (BHC)
• Dichloro diphenyl trichloroethane (DDT)
The key notes of the chapter are as follows:
Polyhalogen Compounds
Carbon compounds containing more than one halogen atom are known as polyhalogen compounds. For example: CFCl3, CH2Cl2 etc.
Some important polyhalogen compounds are described below:
1. Dichloromethane, CH2Cl2
Preparation:
Dichloromethane is prepared by the direct chlorination of CH4.
![]()
Properties:
• It is a colourless, sweet-smelling, volatile liquid
• It has a low boiling point and low inflammability.
• It is insoluble in water
Uses:
• It is widely used as a paint remover
• It is used as a solvent and cleaning agent in chemical manufacture, textiles, electronics, metals and plastic industries
• It is also used as a process solvent in the manufacture of drugs
2. Trichloromethane, CHCl3 (Chloroform)
Preparation:
CHCl3 is manufactured by chlorination of methane followed by separation by fractional distillation.
![]()
Properties:
• Chloroform is a sweet – smelling liquid
• Chloroform is slowly oxidised by air in the presence of light to form poisonous phosgene gas
![]()
To avoid this oxidation chiorofrom is always stored in dark coloured bottles filled to the brim to exclude any air. Further bottles are also filled with small amount of ethyl alcohol so as to destroy traces of phosgene if formed, to harmless diethyl carbonate.
![]()
Some important chemical reactions of chloroform are:
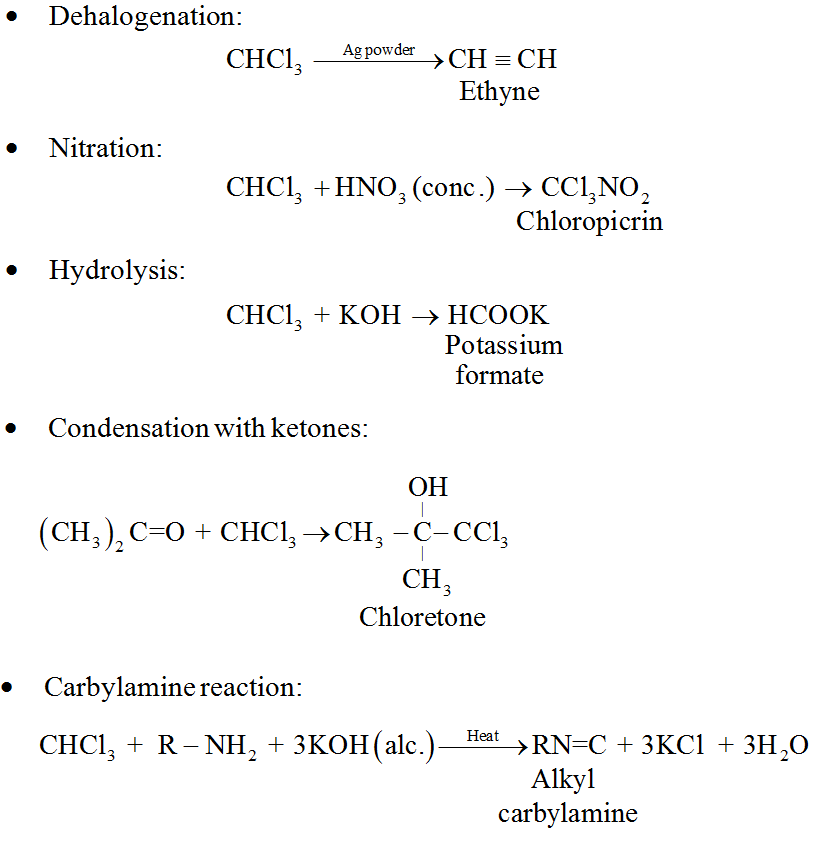
Uses:
• Chloroform is employed as a solvent for fats, alkaloids, iodine and other substances.
• It mainly used in the production of the freon refrigerant R-22.
3. Triiodomethane, CHI3 (Iodoform)
Preparation:
Any compound containing CH3CO− or CH3CH(OH)− group, when heated with iodine and aquous NaOH gives yellow precipitate of iodoform.The reaction is known as iodoforin reaction.
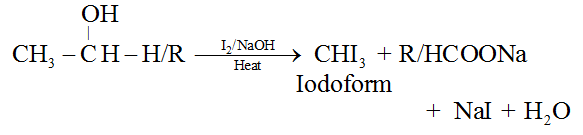
Iodoform can also be prepared conveniently by using bleaching powder, CaOCl2.
![]()
Iodoform was used earlier as an antiseptic due to the liberation of free iodine but due to its objectionable smell, it has been replaced by other formulations containing iodine.
4. Tetrachloromethane, CCl4 (Carbon tetrachloride)
Preparation:
CCl4 is prepared by reacting carbon disulphide (CS2) with Cl2 in the presence of AlCl3.
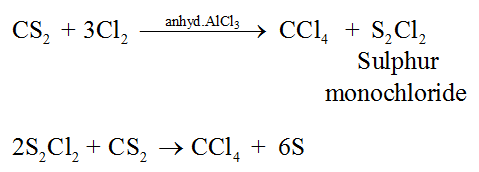
Properties:
• Iodoform is a colourless, non-inflammable, poisonous liquid.
• When heated with steam at 773 K, it undergoes oxidation to form phosgene, COCl2 gas.
![]()
Uses:
• Carbon tetrachloride is used as a solvent for oils, fats, resins
• It is used as a refrigerant and a dry cleaning agent
• It is used as a fire extinguisher under the name pyrene
Note: When carbon tetrachloride is released into the air, it causes the atmospheric temperature to rise and depletion of ozone layer. Depletion of the ozone layer is believed to increase human exposure to ultraviolet rays, leading to increased skin cancer, eye diseases and disorders, and possible disruption of the immune system.
5. Freons
Freons are the chlorofluoro compounds of methane and ethane.
Preparation:
Dichloro difluoro methane or Freon 12 (CCl2F2) is one of the most common freons in and is manufactured from tetrachloromethane by Swarts reaction
![]()
Properties:
• Freons are extremely stable, unreactive, non-toxic, non-corrosive and easily liquefiable gases
• They lead to the depletion of ozone layer surrounding our planet
Uses:
• Freons are used in aerosol propellants, refrigeration and air conditioning purposes
6. Benzene hexachloride (BHC)
It is also famous by its trade name, gammaxene or lindane
Preparation:
It is prepared by reacting benzene with chlorine in the presence of sunlight.
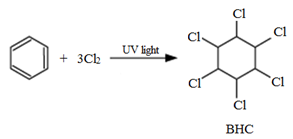
Uses:
BHC is mainly sused as a pesticide in agriculture.
7. Dichloro diphenyl trichloroethane (DDT)
Preparation:
It is manufactured by the condensation of chloral with chlorobenzene in the presence of H2SO4.
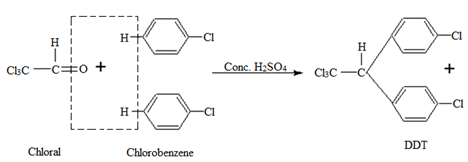
Properties:
• DDT is a white powder insoluble in water.
• It is a highly stable fat-soluble compound.
Uses:
• It is mainly used as an insecticide.
Haloalkanes and Haloarenes MCQ Chapter 10
The Haloalkanes are a group of chemical compounds having an alkane with one or more hydrogen replaced by a halogen atom (group 17 elements). The difference between an alkane and haloalkane is quite significant.
The structural difference is because of the replacement of one or more hydrogen with a halogen atom. The difference in physical property is due to the difference in the electronegativity, bond length, bond strength, molecular size etc.
Haloarenes are aromatic compounds in which the halogen atom attaches directly to the carbon atom of the aromatic ring.
Below are some of the very important NCERT Haloalkanes and Haloarenes MCQ Class 12 Chemistry Chapter 10 with Answers. These Haloalkanes and Haloarenes MCQs have been prepared by expert teachers and subject experts based on the latest syllabus and pattern of CBSE Term 1 examination.
Haloalkanes and Haloarenes Class 12 Chemistry MCQs
1. Which of the following undergoes nucleophilic substitution exclusively by SN 1 mechanism?
(a) Benzyl chloride
(b) Ethyl chloride
(c) Chlorobenzene
(d) Isopropyl chloride
Answer/Explanation
Answer: a
Explaination:
(a) It is because benzyl carbocation is stabilised by resonance.
2. The increasing order of nucleophilicity would be
(a) Cl– < Br– < I–
(b) I– < Cl– < Br–
(c) Br– < Cl– < F–
(d) I– < Br– < Cl–
Answer/Explanation
Answer: a
Explaination:
(a) l– is best nucleophile because it can donate electron easily.
3.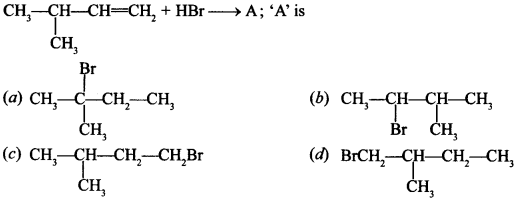
Answer/Explanation
Answer: a
Explaination:
(a) ∵ 3° carbocation is most stable.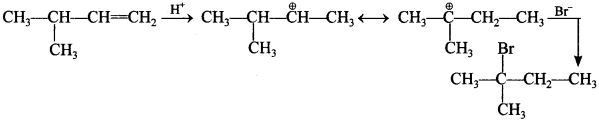
4. Which of the following is most reactive towards SN1 reaction?
(a) C6H5C(CH3)C6H5Br
(b) C6H5CH2Br
(c) C6H5CH(C6H5)Br
(d) C6H5CH(CH3)Br
Answer/Explanation
Answer: a
Explaination:
(a) Since it is 3° halide, carbocation is stabilised by resonance.
5. The correct order of increasing the reactivity of C—X bond towards nucleophile in following compounds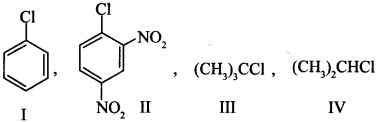
(a) IV < III < I < II
(c) I < II < IV < III
(b) III < II < I < IV
(d) II < III < I < IV
Answer/Explanation
Answer: c
Explaination:
(c) III is most reactive due to stability of 3° carbocation, -NO2 (electron withdrawing) group increase nucleophilic substitution reaction.
6. m-Xylene reacts with Br2 in presence of FeBr3, what are products formed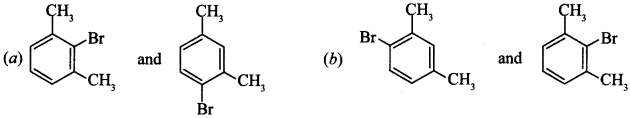
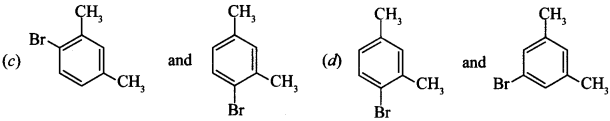
Answer/Explanation
Answer: c
Explaination:
(c) It is due to stearic hinderance, these products will be formed, —CH3 group is o andp-directing.
7. Which of the following compound will undergo racemisation when reacts with aq. KOH?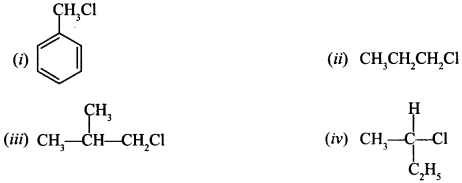
(a) (i) and (ii)
(b) (ii) and (iv)
(c) (iii) and (iv)
(d) (iv)
Answer/Explanation
Answer: d
Explaination:
(d) IV.
Since, it has chiral ‘C’ atom, others do not have.
8.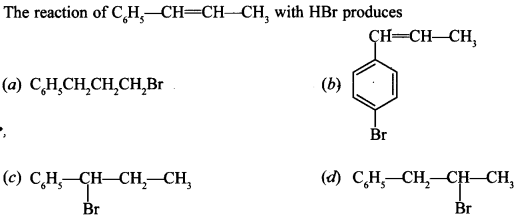
Answer/Explanation
Answer: c
Explaination:
(c) Since, its carbocation is stabilised by resonance as well as +1 effect.
9. CH3CH2CH2Br + NaCN → CH3CH2CH2CN + NaBr, will be fastest in
(a) ethanol
(b) methanol
(c) N, N dimethyl formamide
(d) Water
Answer/Explanation
Answer: c
Explaination: (c) It is favoured by non-polar solvent.
10.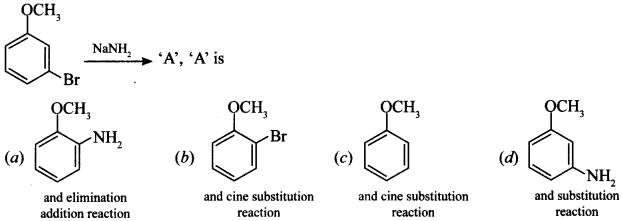
Answer/Explanation
Answer: d
Explaination:
(d) It is substitution reaction but follows benzyne mechanism.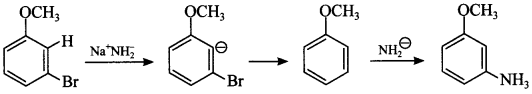
11. A dihalogen derivative ‘X’ of a hydrocarbon with three carbon atoms react with ale. KOH and produces hydrocarbon which forms red ppt. with ammonical Cu2Cl2. ‘X’ gives an aldehyde on reaction with aq. KOH. The compound ‘X’ is
(a) 1, 3-Dichloropropane
(b) 1, 2-Dichloropropane
(c) 2, 2-Dichloropropane
(d) 1, 1-Dichloropropane
Answer/Explanation
Answer: d
Explaination: d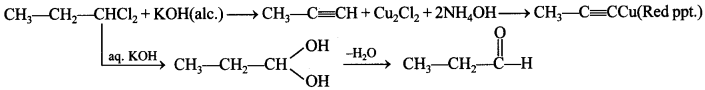
12. The synthesis of alkyl fluoride is best accomplished by
(a) Finkelstein reaction
(b) Swartz reaction
(c) Free radical fluorination
(d) Sandmeyers reaction
Answer/Explanation
Answer: b
Explaination:
(b) C2H5Cl + AgF → C2H5F + AgCl.
13. How many chiral compounds are possible on monochlorination of 2-methyl butane?
(a) 2
(b) 4
(c) 6
(d) 8
Answer/Explanation
Answer:
Explaination: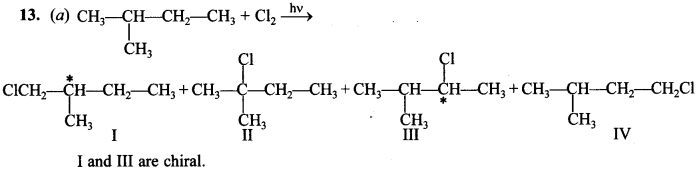
14. The increasing order of reactivity towards SN1 mechanism is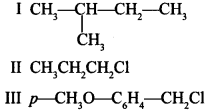
(a) III < II < I
(b) II < I < III
(c) I < III < II
(d) II < III < I
Answer/Explanation
Answer: b
Explaination:
(b) ∵ 1° < 2° < Benzyl carbocation with OCH3 at p-position is order of stability of carbocation.
15. Arrange the following compounds in the increasing order of their densities. [NCERT Exemplar]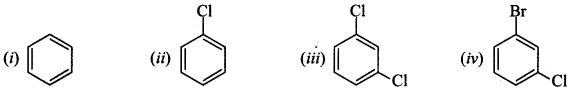
(a) (i) < (ii) < (iii) < (iv)
(b) (i) < (iii) < (iv) < (ii)
(c) (iv) < (iii) < (ii) < (i)
(d) (ii) < (iv) < (iii) < (i)
Answer/Explanation
Answer: a
Explaination: (a) Higher the molecular weight, higher will be density.
16. What is ‘A’ in the following reaction? [NCERT Exemplar]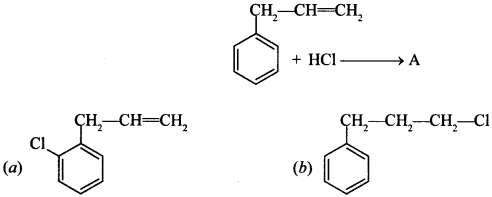

Answer/Explanation
Answer: c
Explaination:
(c) It is MarkownikofFs addition, secondary carbocation is more stable.
17. Which of the following alkyl halides will undergo SN1 reaction most readily? [NCERT Exemplar]
(a) (CH3)3 C—F
(b) (CH3)3 C—Cl
(c) (CH3)3 C—Br
(d) (CH3)3 C—I
Answer/Explanation
Answer: d
Explaination:
(d) It is because C—I bond is weakest due to longer bond length.
18. Which of the carbon atoms present in the molecule given below are asymmetric? [NCERT Exemplar]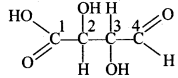
(a) 1, 2, 3, 4
(b) 2, 3
(c) 1, 4
(d) 1, 2, 3
Answer/Explanation
Answer: b
Explaination:
(b) 2 and 3 are chiral as these are linked with four different groups.
19. Which of the following compounds will give racemic mixture on nucleophilic substitution by OH” ion? [NCERT Exemplar]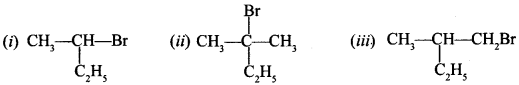
Answer/Explanation
Answer: a
Explaination:
(a) Since it is optically active 2° halide, will follow SN1 mechanism.
20. Which of the following compounds will give racemic mixture on nucleophilic substitution by OH” ion? [NCERT Exemplar]
1-Bromoethane, 1-Bromopropane, 1-Bromobutane, Bromobenzene
(a) Bromobenzene < 1-Bromobutane < 1-Bromopropane < 1-Bromoethane
(b) Bromobenzene < 1-Bromoethane < 1-Bromopropane < 1-Bromobutane
(c) 1-Bromopropane < 1-Bromobutane < 1-Bromoethane < Bromobenzene
(d) 1-Bromoethane < 1-Bromopropane < 1-Bromobutane < Bromobenzene
Answer/Explanation
Answer: d
Explaination:
(d) Higher the molecular weight, higher will be boiling point.
21. SN1 reaction of alkyl halides lead to
(a) Retention of configuration
(b) Racemisation
(c) Inversion of configuration
(d) None of these
Answer
Answer: b
22. p-djchlorobenzene has higher melting point than its o- and m- isomers because
(a) p-dichlorobenzene is more polar than o- and m- isomer.
(b) p-isomer has a symmetrical crystalline structure.
(c) boiling point of p-isomer is more than o- and m-isomer.
(d) All of these are correct reasons.
Answer
Answer: b
23. Chloropicrin is formed by the reaction of
(a) steam on carbon tetrachloride.
(b) nitric acid on chlorobenzene.
(c) chlorine on picric acid.
(d) nitric acid on chloroform.
Answer
Answer: d
24. Fitting reaction can be used to prepare
(a) Toluene
(b) Acetophenon
(c) Diphenyl
(d) Chlorobenzene
Answer
Answer: c
25. Identify the end product (C) in the following sequence: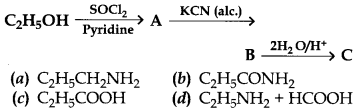
Answer
Answer: c
26.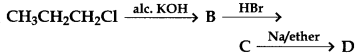
In the above reaction, the product D is
(a) Propane
(b) 2, 3-Dimethylbutane
(c) Hexane
(d) Allyl bromide
Answer
Answer: b
27. Identify X and Y in the following sequence![]()
(a) X = KCN, Y = LiAlH4
(b) X = KCN, Y = H3O+
(c) X = CH3Cl, Y = AlCl3 HCl
(d) X = CH3NH2, Y = HNO2
Answer
Answer: a
28. In the following sequence of reactions:![]()
(a) n-propylamine
(b) isopropylamine
(c) ethylamine
(d) ethylmethylamine
Answer
Answer: d
29.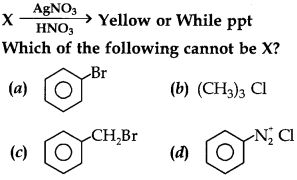
Answer
Answer: a
30.
Identifay Z in the series
(a) C2H5I
(b) C2H5OH
(c) CHI3
(d) CH3CHO
Answer
Answer: c
Note: In the following questions two or more options may be correct. (Q.21 to Q.27)
Consider the following reaction and answer the questions No. 21-23.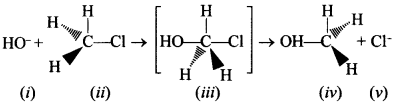
31. Which of the statements are correct about above reaction? [NCERT Exemplar]
(a) (i) and (v) both are nucleophiles.
(b) In (iii) carbon atom is sp3 hybridised.
(c) In (iii) carbon atom is sp2 hybridised.
(d) (i) and (v) both are electrophiles.
Answer/Explanation
Answer:
Explaination:
(a) and (c) are correct.
(i) and (v) are -vely charged
∴ nucleophile. ‘C’ is sp² hybridised as C—OH and C—Cl bonds are half broken and half formed.
32. Which of the following statements are correct about this reaction? [NCERT Exemplar]
(a) The given reaction follows SN2 mechanism.
(b) (ii) and (iv) have opposite configuration.
(c) (ii) and (iv) have same configuration.
(d) The given reaction follows SN1 mechanism.
Answer/Explanation
Answer:
Explaination:
(a) and (b). In SN2 mechanism, inversion of configuration takes place due to back side attack.
33. Which of the following statements are correct about the reaction intermediate? [NCERT Exemplar]
(a) Intermediate (iii) is unstable because in this carbon is attached to 5 atoms.
(b) Intermediate (iii) is unstable because carbon atom is sp² hybridised.
(c) Intermediate (iii) is stable because carbon atom is sp² hybridised.
(d) Intermediate (iii) is less stable than the reactant (ii).
Answer/Explanation
Answer:
Explaination:
(a) and (d), intermediate is unstable than reactant as well as product as ‘C’ is bonded to 5 carbon atoms.
Answer Q. No. 24 and 25 on the basis of the following reaction.
(i) (ii) (iii) (iv)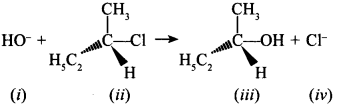
34. Which of the following statements are correct about the mechanism of this reaction? [NCERT Exemplar]
(a) A carbocation will be formed as an intermediate in the reaction.
(b) OH– will attach the substrate (ii) from one side and Cl– will leave it simultaneously from other side.
(c) An unstable intermediate will be formed in which OH– and Cl– will be attached by weak bonds.
(d) Reaction proceeds through SN1 mechanism.
Answer/Explanation
Answer:
Explaination:
(a) and (d) are correct, In SN1 carbocation is formed. OH– can attract freely from either of the side.
35. Which of the following statements are correct about the kinetics of this reaction? [NCERT Exemplar]
(a) The rate of reaction depends on the concentration of only (ii).
(b) The rate of reaction depends on concentration of both (i) and (ii).
(c) Molecularity of reaction is one.
(d) Molecularity of reaction is two.
Answer/Explanation
Answer:
Explaination:
(a) and (d).
∴ Molecularity is 2 and it follows SN1 mechanism, therefore, rate depends upon cone, of 3° halide only.
36. Haloalkanes contain halogen atom (s) attached to the sp3 hybridised carbon atom of an alkyl group. Identify haloalkane from the following compounds. [NCERT Exemplar]
(a) 2-Bromopentane
(b) Vinyl chloride (chloroethene)
(c) 2-chloroacetophenone
(d) Trichloromethane
Answer/Explanation
Answer:
Explaination:
(a) 2-Bromopentane (d) Trichloromethane have sp3 hybridised ‘C’.
37. Ethylene chlonde and ethylidene chloride are isomers. Identify the correct statements. [NCERT Exemplar]
(a) Both the compounds form same product on treatment with alcoholic KOH.
(b) Both the compounds form same product on treatment with aq.NaOH.
(c) Both the compounds form same product on reduction.
(d) Both the compounds are optically active.
Answer/Explanation
Answer:
Explaination: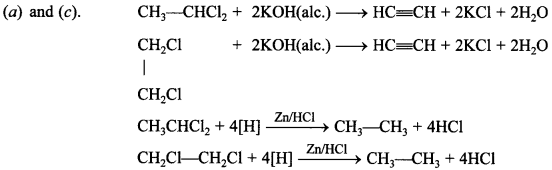
38. Match the the compounds given in Column I with the effects given in Column II. [NCERT Exemplar]
| Column I | Column II |
| (a) Chloramphenicol | (i) Malaria |
| (b) Thyroxine | (ii) Anaesthetic |
| (c) Chloroquine | (iii) Typhoid fever |
| (d) Chloroform | (iv) Goiter |
| (v) Blood substituent |
Answer/Explanation
Answer:
Explaination:
(a) (iii)
(b) (iv)
(c) (i)
(d) (ii)
39. Match the items of Column I and Column II. [NCERT Exemplar]
| Column I | Column II |
| (a) SN1 reaction | (i) vio-dibromides |
| (b) Chemicals in fire extinguisher | (ii) gem-dihalides |
| (c) Bromination of alkenes | (iii) Racemisation |
| (d) Alkylidene halides | (iv) Saytzeffrule |
| (e) Elimination of HX from alkylhalide | (v) Chlorobromocarbons |
Answer/Explanation
Answer:
Explaination:
(a) (iii)
(b) (v)
(c)(i)
(d) (ii)
(e) (iv)
40. Match the reactions given in Column I with the types of reactions given in Column II. [NCERT Exemplar]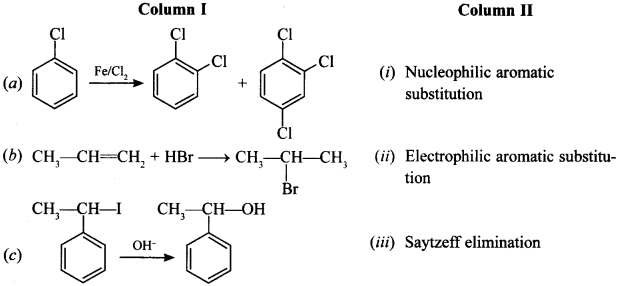
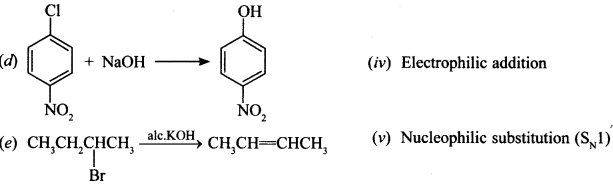
Answer/Explanation
Answer:
Explaination:
(a) Aromatic electrophilic substitution (ii)
(b) Electrophilic addition (iv)
(c) Nucleophilic substitution (v)
(d) Nucleophilic aromatic substitution (i)
(e) Saytzeff elimination (iii)
Note: In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices. (Q.31 to Q.35)
(a) Assertion and reason both are correct and reason is correct explanation of assertion.
(b) Assertion and reason both are wrong statements.
(c) Assertion is correct but reason is wrong statement.
(d) Assertion is wrong but reason is correct statement.
(e) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
41. Assertion: KCN reacts with methyl chloride to give methyl isocyanide.
Reason: CN– is an ambident nucleophile. [NCERT Exemplar]
Answer/Explanation
Answer:
Explaination:
(d) Assertion is wrong but reason is correct statement. AgCN reacts with CH3Cl to give isocyanide, KCN gives cyanide. CN– is ambident nucleophile.
42. Assertion: fert-Butyl bromide undergoes Wurtz reaction to give 2, 2, 3, 3-tetramethylbutane.
Reason: In Wurtz reaction, alkyl halides react with sodium in dry ether to give hydrocarbon containing double the number of carbon atoms present in the halide. [NCERT Exemplar]
Answer/Explanation
Answer:
Explaination:
(a) Assertion and reason both are correct and reason is correct explanation of assertion.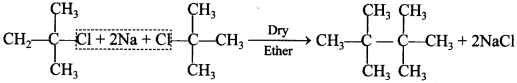
43. Assertion: Presence of a nitro group at ortho or para position increases the reactivity of haloarenes towards nucleophilic substitution.
Reason: Nitro group, being an electron withdrawing group decreases the electron density over the benzene ring. [NCERT Exemplar]
Answer/Explanation
Answer:
Explaination:
(a) Assertion and reason both are correct and reason is correct explanation of assertion.
44. Assertion: In monohaloarenes, further electrophilic substitution occurs at ortho and para positions.
Reason: Halogen atom is a ring deactivator. [NCERT Exemplar]
Answer/Explanation
Answer:
Explaination:
(e) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
45. Assertion: Aryl iodides can be prepared by reaction of arenes with iodine in the presence of an oxidising agent.
Reason: Oxidising agent oxidises I2 into HI. [NCERT Exemplar]
Answer/Explanation
Answer:
Explaination:
(c) Assertion is correct but reason is wrong statement.
46. Chloromethane on treatment with excess of ammonia gives __________ .
Answer/Explanation
Answer:
Explaination: Methanamine
47. The isomer of C4H9Br, (optical active) is __________ .
Answer/Explanation
Answer:
Explaination: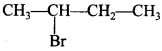
48. Out of chlorobenzene, o-chlorotoluene, m-chloro toluene, least reactive towards nucleophilic substitution is __________ .
Answer/Explanation
Answer:
Explaination:
o-Chlorotoluene, because—CH3 group is electron releasing, decreases reactivity towards nucleophilic substitution reactions.
49.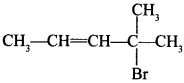
is allylic halide. [True or False]
Answer/Explanation
Answer:
Explaination: True.
50. When benzene reacts with Cl2 and FeCl3, the attacking electrophile is Cl+. [True or False]
Answer/Explanation
Answer:
Explaination:![]()
51. IUPAC name of Diethyl bromomethane is 3-Bromo-pentane.
Answer/Explanation
Answer:
Explaination: True.
52. Among isobutyl bromide, n-Butyl bromide, secondary butyl bromide and tertiary butyl bromide,
n-Butyl bromide has lowest boiling point. [True or False]
Answer/Explanation
Answer:
Explaination: False, it has highest boiling point 3° < isobutyl bromide < 2° < 1°.
53. Reactivity order of HI > HBr > HCl > HBr towards nucleophilic substitution reaction. [True or False]
Answer/Explanation
Answer:
Explaination: True. HI has lowest bond dissociation enthalphy.
54. Tert. halides undergo elimination reaction faster than nucleophilic substitution reaction. [True or False]
Answer/Explanation
Answer:
Explaination: True. Tertiary halides form carbocation followed by elimination.
55. Write the IUPAC name of the following compound:
CH2 =CHCH2Br [AI2011]
Answer/Explanation
Answer:
Explaination: 3-Bromoprop-1-ene.
56. Give the IUPAC name of the following compound. [Delhi 2012]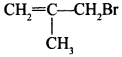
Answer/Explanation
Answer:
Explaination: 3-Bromo-2-methylprop-1 -ene.
57. Write the IUPAC name of the following compound: (CH3)3CCH2Br [Delhi 2011]
Answer/Explanation
Answer:
Explaination: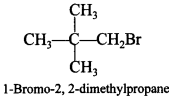
58. Write the IUPAC name of the following compound: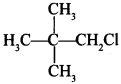
Answer/Explanation
Answer:
Explaination: 1-Chloro-2, 2-dimethylpropane.
59. Write IUPAC name of the given compound: [Delhi 2019]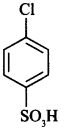
Answer/Explanation
Answer:
Explaination: 4-chloro benzene sulphonic acid.
60. Draw the structure of 2-Bromopentane [Delhi 2014(C)]
Answer/Explanation
Answer:
Explaination: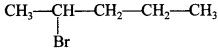
61. Write the structure of the following compound:
2 -(2-chlorophenyl)-l-iodoethane. [AI 2011(C)]
Answer/Explanation
Answer:
Explaination: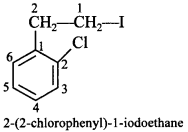
62. How can you obtain iodoethane from ethanol when no other iodine containing reagent except Nal is available in the laboratory?
Answer/Explanation
Answer:
Explaination: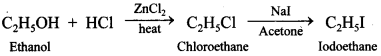
63. What happens when bromine attacks![]()
Answer/Explanation
Answer:
Explaination:
64. Identify the products A and B formed in the following reaction:![]()
Answer/Explanation
Answer:
Explaination: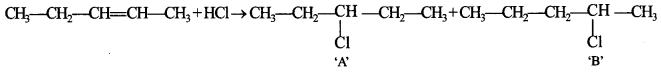
65. How will you convert methyl iodide from methyl bromide? What is the name of this reaction?
Answer/Explanation
Answer:
Explaination:
This is Finkelstein reaction.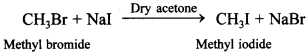
66. Arrange the following haloalkanes in the increasing order of their densities:![]()
Answer/Explanation
Answer:
Explaination:
n-C3H7Cl < n-C3H7Br < n-C3H7I (Increasing order of density)
67. Which would undergo SN2 reaction faster in the following pair and why? [Delhi 2015]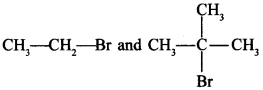
Answer/Explanation
Answer:
Explaination:
CH3CH2Br will react faster due to less stearic hindrance.
68. Which would undergo SN1 reaction faster in the following pair: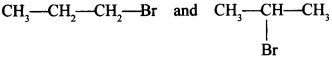
Answer/Explanation
Answer:
Explaination: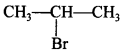
will react faster due to 2°halide, will give stable carbocation.
69. Why is f-butyl bromide more reactive towards SN1 reaction as compared to n-butyl bromide? [Uttarakhand 2019]
Answer/Explanation
Answer:
Explaination:
It is because tert. butyl carbocation is more stable than n-butyl carbocation.
70. Which would undergo SN2 reaction faster in the following pair and why? [Foreign 2015]
CH3—CH2—Cl or C6H6CH2Cl
Answer/Explanation
Answer:
Explaination:
CH3CH2l will undergo SN2 reaction faster because it has lower bond dissociation enthalpy of C—I bond due to longer bond length, r is better leaving group.
71. Which would undergo SN1 reaction faster in the following pair and why? [AI 2015]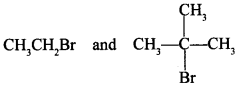
Answer/Explanation
Answer:
Explaination: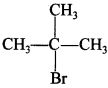
because it forms stable 3° carbocation.
72. Which alkyl halide from the following pair would you expect to react more rapidly by an SN2 mechanism? [Uttarakhand 2019]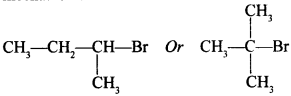
Answer/Explanation
Answer:
Explaination:
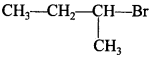
will react faster because it has less stearic hindrance.
73. Which will react faster in SN2 displacement, 1-bromopentane or 2-bromopentane, and why? [Foreign 2011]
Answer/Explanation
Answer:
Explaination:
1-Bromopentane will react faster in SN2 because it is 1° (primary) halide and has less stearic hindrance.
74. A solution of KOH hydrolyses CH3CHClCH2CH3 and CH3CH2CH2CH2Cl. Which one of these is more easily hydrolysed?
Answer/Explanation
Answer:
Explaination:
CH3—CH(Cl)CH2CH3 will be more easily hydrolysed because it will form secondary carbocation which is more stable than primary carbocation.
75. Which of the compounds will react faster in SN1 reaction with the OH– ion?
CH3—CH2—Cl or C6H5—CH2—Cl
Answer/Explanation
Answer:
Explaination:
C6H5CH2Cl will react faster due to formation of resonance stabilised carbocation.
76. Explain why the following pairs of compound do not show optical activity.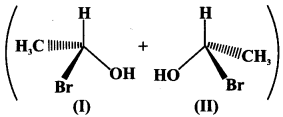
Answer/Explanation
Answer:
Explaination:
It is racemic mixture which contains equal amounts of dextro and laevorotatory substances. If half of the total number of molecules rotates the plane-polarised light towards left, remaining half of the molecules rotates towards right such that net optical rotation is zero.
77. Identify the chiral molecule in the following pair: [AI 2014]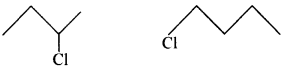
Answer/Explanation
Answer:
Explaination:![]()
78. What is meant by enantiomers?
Answer/Explanation
Answer:
Explaination:
The stereoisomers which are non-superimposable mirror images are called enantiomers, e.g. d(+) glucose and l(-) glucose are enantiomers.
79. What is the major product formed from dehydrohalogenation of 2-Bromopentane?
Answer/Explanation
Answer:
Explaination:
Pen-2-ene (81%)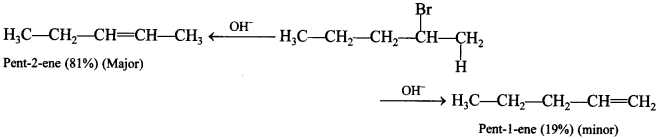
80. Give the chemical reaction involved in the formation of Grignard reagent. [Foreign 2011]
Answer/Explanation
Answer:
Explaination: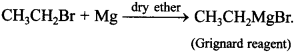
Haloalkanes and Haloarenes MCQ (1-10)
- In the presence of peroxide hydrogen chloride and hydrogen iodide do not give anti-Markovnikov’s addition to alkenes because
- Both are highly ionic
- One is oxidising and the other is reducing
- One of the steps is endothermic in both the cases
- All the steps are exothermic in both the reaction.
Answer/Explanation
Answer: 3
Explanation:The addition of Cl and I radicals to alkanes in an anti-Markovnikov’s fashion is an endothermic reaction and therefore counterproductive. Without the presence of peroxide, all hydrogen halides will add according to the Markovnikov rule
- On being heated with aniline and KOH, chloroform gives
- An almond like smell
- A rose-like odor
- A smell like oil of wintergreen
- An offensive smell
Answer/Explanation
Answer: 4
Explanation:
Carbylamine reaction takes place
C6H5NH2 + CHCl3 + 3KOH → C6H4NC + 3KCl + 3H2O.As isocyanide is formed, an offensive smell is given out
- Choose the incorrect statement
- An SN1 reaction proceeds with inversion of configuration
- An SN2 reaction proceeds with stereochemical inversion
- An SN2 reaction follows second order kinetics
- The reaction of tert-butyl bromide with OH– follows first order kinetics
Answer/Explanation
Answer: 1
Explanation:
SN1 reaction proceeds with racemisation and retention of configuration.
- Which of the statements is not true for the isomeric compounds ethylene chloride and ethylidene chloride?
- Both react with aqueous KOH to give the same product
- Both react with alcoholic KOH to give the same product
- They are derivatives of ethene
- They respond to Beilstein’s test
Answer/Explanation
Answer: 1
Explanation:
CH2(Cl)-CH2(Cl) + aq KOH → CH2(OH)-CH2(OH).Ethylene dichloride
CH3-CH(Cl)2 + aq KOH → CH3-CHO
Ethylidene chloride
- The reagent for the following conversion is
1 . Alcoholic KOH
2. Alcoholic KOH followed by NaNH2
3. Aqueous KOH followed by NaNH2
4. Zn/CH3OH
Answer/Explanation
Answer: 2
Explanation: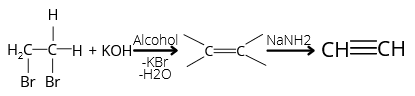 .
.
- Which of the following halogen exhange reaction will occur?
- R-I + NaCl
- R-F + KCl
- R-Cl + NaI
- CH3-F + AgBr
Answer/Explanation
Answer: 3
Explanation:
Bromides and chlorides are insoluble in acetone and hence they can’t form a solution with acetone to react with alkyl halides. Sodium iodide, however is soluble in acetone thus forms a solution with acetone and reacts with alkyl halides, thus undergoing Finkelstein reaction.R-X + NaI → R-I + NaX
- Which of the following groups are ortho and para directing?
- -CHO
- -NHCOCH3
- -CN
- -SO3H
Answer/Explanation
Answer: 4
Explanation:
-SO3H is an ortho and para directing group..
- The trade name of trochloroethylene is
- Freon
- Westron
- Westrosol
- DDT
Answer/Explanation
Answer: 3
Explanation:
The trade name of trichloroethylene is westrosol..
- The IUPAC name of the compound shown below is
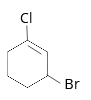
1 . 2-bromo-6-chlorocyclohex-1-ene
2. 6-bromo-2-chlorocyclohexene
3. 3-bromo-1-chlorocyclohexene
4. 1-bromo-3-chlorocyclohexene
Answer/Explanation
Answer: 3
Explanation:
3-bromo-1-chlorocyclohexene.
10. The increasing order of hydrolysis of the following compounds is
1 . a < d < b < c 2. a < b < c < d
3. a < b < d < c 4. d < c < b < a
Answer/Explanation
Answer: 2
Explanation:
a < b < c < d
- On being warmed with silver powder, chloroform gives
- C6H6
- C2H4
- C2H2
- CH3Cl
Answer/Explanation
Answer: 3
Explanation:
2CHCl4 + 6Ag → C2H2 (ethyne) + 6 AgCl.
- The addition of propene with HOCl proceeds via the additions of
- H+ in the first step
- Cl– in the first step
- OH– in the first step
- Cl+ and OH– in a single step
Answer/Explanation
Answer: 2
Explanation:
Alkenes undergo electrophilic addition reactions. HOCl on self ionisation produces Cl+ which attacks 1st.HOCl + HOCl → H2O + OCl– + Cl+
- Chlorobenzene can be obtained from benzenediazonium chloride by
- Friedel-Crafts reaction
- Sandmeyer reaction
- Wurtz reaction
- Fittig reaction
Answer/Explanation
Answer: 2
Explanation:
The formation of chlorobenzene by the reaction of benzene diazonium chloride with Cu2Cl2/HCl is known as Sandmeyer’s reaction..
- Which of the following reactions cannot be used for the preparation of alkyl halides?
- CH3CH2OH + HCl →
- CH3CH2OH + HCl + anhy. ZnCl →
- (CH3)3COH + HCl →
- (CH3)2CHOH + HCl →
Answer/Explanation
Answer: 1
Explanation:
Alkyl halide is prepared by the reaction of an alcohol with HCl in which the -OH group is replaced with Cl. Primary and secondary alcohols require catalysts such as anhydrous zinc chloride. Tertiary alcohols do not require such catalysts as they are more reactive.
- Butyronitrile can be prepared by heating
- Propyl alcohol with KCN
- Butyl alcohol with KCN
- Propyl chloride with KCN
- Butyl chloride with KCN
Answer/Explanation
Answer: 3
Explanation:
Butyronitrile can be prepared by heating propyl chloride with KCN.CH3-CH2-CH2-Cl + KCN → CH3-CH2-CH2-CN
- For a given alkyl group, the boiling point of alkyl halides follows the order
- RCl > RBr > RI
- RI > RBr > RCl
- RI > RCl > RBr
- RBr > RI > RCl
Answer/Explanation
Answer: 2
Explanation:
Higher the mass of halogen atoms attached with the hydrocarbon part, higher will be the boiling point. Therefore, as I has the highest mass, then Br and then Cl, the boiling point will decrease as : RI > RBr > RCl.
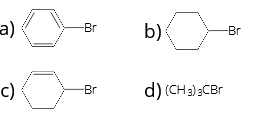
- Which of the following is the most reactive towards nucleophilic substitution reaction?
1 . (a) 2. (b) 3. (c) 4. (d)
Answer/Explanation
Answer: 4
Explanation:![]()
- Addition of HBr gives the same product in the presence or absence of peroxide when alkene is
- 1-butene
- 2-methylpropene
- 1-propene
- 2-butene
Answer/Explanation
Answer: 4
Explanation:
2-butene is a symmetric compound and will produce the same product with HBr with or without peroxide.
- Br has a low reactivity in CH2CHBr because
- The C-Br bond has a partial triple bond character
- Of the +M effect of bromine
- Br is electronegative
- None of the above
Answer/Explanation
Answer: 2
Explanation:
Br has a low reactivity in CH2CHBr because of the +M effect of bromine.
- Which of the following is the most suitable for the preparation of n-propylbenzene?
- Friedal-Crafts Alkylation
- Wurtz Reaction
- Wurtz-Fittig Reaction
- Grignard Reaction
Answer/Explanation
Answer: 3
Explanation:
The most suitable way for preparing n-propylbenzene is wurtz-fittig reaction
- What is the hybridisation of carbon that is attached to chlorine atom in n-primary alkyl halide?
- sp3
- sp2
- sp
- sp3d
Answer/Explanation
Answer: 1
Explanation:
primary alkyl halide is R – CH2 – X. In alkyl halides, halogen is always attached to an sp3 carbon.
- The C-X bond is strongest in
- CH3Cl
- CH3Br
- CH3F
- CH3I
Answer/Explanation
Answer: 3
Explanation:
The electron pair is pulled towards the halogen in the carbon-halogen bond as the halogen is more electronegative. As fluorine is the most electronegative element, CH3F has the strongest bond and has the shortest bond length.
- IUPAC name for tertiary butyl iodide is
- 4-iodobutane
- 2-iodobutane
- 1-iodo-3-methylpropane
- 3-iodo-2-methylpropane
Answer/Explanation
Answer: 4
Explanation:
Tertiary butyl iodide is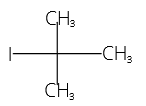
IUPAC Name = 2-iodo-2-methyl propane
- Select the incorrect statement
- 1,4-dibromobutane react with excess of magnesium in ether to generate di-Grignard reagent
- 1,2-dichlorocyclohexane treated with excess of magnesium in ether produces cyclohexane
- Vicinal dihalides undergo dehalogenation to give alkene when heated with Zn dust or Mg
- 1,3-dichloropropane by treatment with Zn dust or Mg forms cyclopropane
Answer/Explanation
Answer: 3
Explanation:
Wrong statement – Vicinal dihalides undergo dehalogenation to give alkene when heated with Zn dust or Mg.
- The reaction of benzene with chlorine in the presence of iron gives
- Benzene hexachloride
- Chlorobenzene
- Benzyl chloride
- Benzoyl chloride
Answer/Explanation
Answer: 2
Explanation: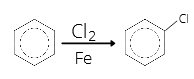
- Which is used as an anaesthetic during surgery?
- Chloroquine
- Thyroxine
- Halothane
- Chloramphenicol
Answer/Explanation
Answer: 3
Explanation:
Chloroquine – used for malaria. Thyroxine – hormone of thyroid gland . Halothane – anesthetic during surgery . Chloramphenicol – antibiotic for bacterial infections
- Which among the following halides will acquire colour when exposed to light?
- R-Br and R-Cl
- R-I and R-F
- R-Cl and R-Br
- R-Br and R-I
Answer/Explanation
Answer: 4
Explanation:
As we move down the group from fluorine to iodine, molecular size increases..
- Which of the following is used in paint removal?
- CHCl3
- CH2Cl2
- CCl4
- CH3Cl
Answer/Explanation
Answer: 2
Explanation:
CHCl3 is used as a solvent and as an anaesthetic. CCl4 is used as a fire extinguisher. CH3Cl is used as a refrigerator
- Ethyl chloride is converted to butane by
- Kolbe’s synthesis
- Williamson synthesis
- Wurtz reaction
- Gatterman reaction
Answer/Explanation
Answer: 3
Explanation:
Wurtz reaction : RX + 2Na + RX → R-R + 2NaX
- Aryl halide is less reactive than alkyl halide towards nucleophilic substitution because
- Of less stable carbonium ion
- Due to large C-Cl bond energy
- Of inductive effect
- Of resonance stabilization and sp3 hybridisation of C attached to halide
Answer/Explanation
Answer: 4
Explanation:
Aryl halides are less reactive towards nucleophilic substitution reaction as compared to alkyl halides due to resonance stabilisation. Due to resonance, C-Cl bond acquires partial double bond character and becomes shorter and stronger and can’t be easily replaced by nucleophiles.
31. Identify B in the following reaction
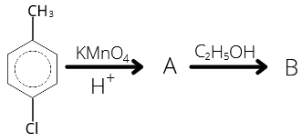
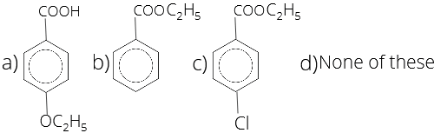
1 . (a) 2. (b) 3. (c) 4. (d)
Answer/Explanation
Answer: 3
Explanation:
CH3-C6H5-Cl + KMnO4 → COOH-C6H5-Cl + C2H5COOH → COOC2H5-C6H5-Cl.
- Which metal is used in Wurtz synthesis?
- Barium
- Aluminium
- Sodium
- Iron
Answer/Explanation
Answer: 3
Explanation:
Sodium metal is used in the Wurtz reaction..
- The false statement about alkane is
- This does not perform polymerisation reaction
- This doesn’t gives elimination reaction
- It doesn’t disappear the colour of dilute KMnO4 solution
- It does not decolourize bromine water
Answer/Explanation
Answer: 2
Explanation:
Alkanes do show elimination reactions.
- Which of the following is used to decaffeinate coffee?
- Iodoform
- Carbon tetrachloride
- Methylene dichloride
- Chloroform
Answer/Explanation
Answer: 3
Explanation:
Methylene dichloride is used to decaffeinate coffee.
- Which of the following statements is false about chloroform?
- It is a colourless, sweet-smelling liquid
- It is almost insoluble in water
- It can be used as an inhalational anaesthetic agent
- It is highly inflammable
Answer/Explanation
Answer: 4
Explanation:
Chloroform is not highly inflammable.
- Which if the following can not be precipitated with an aqueous solution of AgNO3?
- CHCl3
- KI
- K2SO4
- KCl
Answer/Explanation
Answer: 1
Explanation:
In CHCl3, there are no free Cl– ions, hence. It doesn’t form a precipitate with an aqueous solution of AgNO3.
- Reaction of chlorobenzene with CH3Cl in presence of AlCl3 gives
- Toluene
- m-chloro toluene
- only o-chloro toluene
- Mixture of ortho and para chlorotoluene
Answer/Explanation
Answer: 4
Explanation:
The Cl– group attached is an ortho-para directing group, therefore, it will form ortho and para chloro toluene.
- On nitration, the major product that chlorobenzene gives is
- 1-chloro-4-nitrobenzene
- 1,4-dinitrobenzene
- 1-chloro-3-nitrobenzene
- 2,4,6-trinitrobenzene
Answer/Explanation
Answer: 2
Explanation:
As Cl- is an ortho-para directing group, it will form ortho-para nitrobenzene.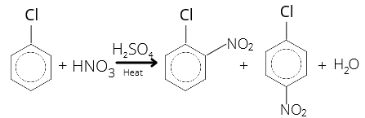
- Common name of chloroethane is
- Benzyl chloride
- Vinyl chloride
- Allyl bromide
- None of these
Answer/Explanation
Answer: 2
Explanation: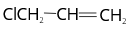
The substituent group attaches to the double bonded or sp2 hybridised carbon. Therefore, it is called vinyl chloride.
- Which if the following has the highest reactivity?
- CH3Br
- CH3CH2Br
- CH3CH2CH2Br
- CH3CH2CH2CH2Br
Answer/Explanation
Answer: 1
Explanation:
Br is an electronegative element. The compound is stable if the carbon chain is of more length and thereby inductive effect increases. CH3Br is less stable and shows higher reactivity because of less inductive effect. Other options have more than one carbon and hence inductive effect increases, stability increases and reactivity decreases.
- Which of the following molecules have the highest dipole moment?
- CH3Cl
- CH2Cl2
- CHCl3
- CCl4
Answer/Explanation
Answer: 1
Explanation:
Methane has a zero dipole moment. Replacement of one H- atom by Cl atom increases the dipole moment. The increase in dipole moment is because the bond dipole moment of C-H bond and that of C-Cl bond reinforce each other.Replacement of another H atom by Cl increases the bond angle due to lone pair-lone pair repulsion between Cl- atoms. This reduces the dipole moment and introduces the third Cl- atom. So when the fourth Cl- atom is introduced, the molecule (CCl4) again becomes symmetrical and the dipole moment reduces to zero. So, CH3Cl has the highest dipole moment..
- Which of the following have the highest boiling point?
- 1-chloropentane
- 2-chloropentane
- 3-chloropentane
- All have equal boiling point
Answer/Explanation
Answer: 1
Explanation:
The more branched a molecule is, the less will be the surface area to interact through van der waal forces and hence, the boiling point will also decrease. Therefore, 1-chloropentane will have the highest boiling point..
- Chlorobenzene and benzene hexachloride are obtained from benzene by the reaction of chlorine, in the presence of
- Direct sunlight and anhydrous AlCl3 respectively
- Sodium hydroxide and sulphuric acid respectively
- Ultraviolet light and anhydrous FeCl3 respectively
- Anhydrous AlCl3 and direct sunlight respectively
Answer/Explanation
Answer: 4
Explanation:
Benzene on substitution reaction with chlorine in the presence of anhydrous AlCl3 will form chlorobenzene. Benzene in addition reaction with chlorine in the presence of sunlight will form benzene hexachloride..
- Which of the following can not be prepared by Sandmeyer’s reaction?
- Chlorobenzene
- Bromobenzene
- Iodobenzene
- All of these
Answer/Explanation
Answer: 3
Explanation:
Sandmeyer’s reaction is used for the preparation of chlorobenzene and bromobenzene..
- Which of the following reagent is used in the conversion of benzene diazonium chloride to chlorobenzene?
- CuCl2
- Cu2Cl2
- FeCl2
- FeCl3
Answer/Explanation
Answer: 2
Explanation:
Cu2Cl2 is used as a reagent and the reaction is called Sandmeyer’s reaction.
- The conversion of ethyl bromide to ethyl iodide using sodium iodide and dry acetone, this reaction is known as
- Swarts reaction
- Finkelstein reaction
- Sandmeyer’s reaction
- Stephen reaction
Answer/Explanation
Answer: 2
Explanation:
The conversion of ethyl bromide to ethyl iodide using sodium iodide and dry acetone, this reaction is known as Finkelstein reaction.C2H5-Br + NaI → C2H5I +NaBr
- The vapours of which of the following compound burns with green coloured flame?
- CH3COCH3
- C2H5OH
- CHCl3
- CH3CHO
Answer/Explanation
Answer: 3
Explanation:
Chloroform burns with green flames gas.
- At normal temperature iodoform is
- Thick viscous liquid
- Gas
- Volatile liquid
- Solid
Answer/Explanation
Answer: 4
Explanation:
At room temperature, iodoform is a yellow coloured solid.
- Iodoform on reaction with KOH gives
- CH3CHO
- HCOOK
- CH3COOK
- HCHO
Answer/Explanation
Answer: 2
Explanation:
CHI3 + 4KOH → HCOOK + 3KCl +2H2O
- Triiodomethane has antiseptic property because of
- Liberation of iodoform
- Liberation of free iodide
- Formation of phosgene gas
- None of these
Answer/Explanation
Answer: 2
Explanation:
Triiodomethane is known as iodoform. As iodoform comes in contact with the matter of skin it decomposes to give free iodine which acts as an antiseptic. It is used for treating skin infection, cuts, boils etc.
Assertion and Reasoning MCQs
Codes
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true and but R is not a correct explanation of A
(c) A is true but R is false
(d) A is false, but R is true
1. Assertion (A) (CH3)3C-O-CH3 give (CH3)3C-I and CH3OH on treatment with HI.
Reason (R) The reaction occurs by SN1 mechanism.
Answer/Explanation
1. (a)
SN1 reaction occurs in compound that has steric hindrance and (CH3)3-C-OCH3 has very much hindrance to attack by reagents.
2. Assertion (A) Hydrogen iodide readily reacts with alkenes to form alkyl halides.
Reason (R) Aqueous hydrohalogen acids are used to prepare alkyl halides from alkenes.
Answer/Explanation
2. (c)
Dry gaseous hydrohalogenation acids are better electrophiles. In aqueous solution, H2O acting as nucleophile may produce alcohol.
3. Assertion (A) CHCI3 is stored in dark bottles.
Reason (R) CHCl3 is oxidized in dark.
Answer/Explanation
3. (c)
CHCl3 is stored in dark bottles to avoid reaction in the presence of light. CHCl3 in the presence of light gets oxidised by air.
4. Assertion (A) CCI4 is a fire extinguisher.
Reason (R) CCI4 is insoluble in water.
Answer/Explanation
4. (b)
CCl4 is carbon tetrachloride and is used in fire extinguisher because it is a heavy non-combustible liquid. CCl4 in insoluble in water due to absence of hydrogen atom that can form hydrogen bonding with water.
5. Assertion (A) CH2-CH-CH2-X is an example of of a allyl halides.
Reason (R) These are the compounds in which the halogen atom is bonded to an sp² hybridised carbon atom.
Answer/Explanation
5. (c)
Allyl halides are the compounds in which the halogen atom is bonded to an sp3 hybridised carbon atom next to carbon-carbon double bond.
6. Assertion (A) Alkylbenzene is not prepared by Friedel-craft alkylation of benzene.
Reason (R) Alkyl halides are less reactive than aryl halides.
Answer/Explanation
6. (c)
Aryl halides are more stable and less reactive due to resonance where the lone pair of electron are in conjugation with a pi bond.
7. Assertion (A) Aryl halides cannot be prepared by replacement of of hydroxyl group of phenol by halogen atom.
Reason (R) Phenol react with halogen acids violently.
Answer/Explanation
7. (c)
Aryl halides can’t be prepared by replacing hydroxyl group of phenols because the carbon oxygen bond in phenols because has a partial double bond character and is difficult to break being stronger than a single bond.
8. Assertion (A) Exposure of ultraviolet rays to human causes the skin cancer, disorder and disrupt the immune system.
Reason (R) Carbon tetrachloride is released into air it rises to atmosphere and deplete the ozone layer.
Answer/Explanation
8. (b)
The ozone layer is depleted as carbon tetrachloride rises into the atmosphere rises into the atmosphere. As the ozone layer depletes, human being are exposed to more UV radiation, which leads to an
9. Assertion (A) The boiling points of alkyl halides decreases in order: RI > RBr > RCI > RF.
Reason (R) The boiling points of alkyl chloride, bromides and iodides are considerably higher than that of the hydrocarbon of comparable molecules mass.
Answer/Explanation
9. (b)
The boiling point of the identical hydrocarbon component is determined by the atomic mass of the halogen atom. The boiling point of a halogen atom increases as its mass increases. As a result, the boiling point of halogen atoms lowers as the atomic mass of the halogen atom decreases.
10. Assertion (A) Electron withdrawing groups in aryl halides increase the reactivity towards nucleophilic substitution
Reason (R) 2, 4 Dinitrochlorobenzene is less reactive than chlorobenzene
Answer/Explanation
10. (c)
When electron withdrawing groups are present at the ortho/para position, halobenzenes become reactive to nucleuphile substitution reaction. This is evident by the fact that 2,4-dinitrochlorobenzene requires milder hydrolysis condition than chlorobenzene.
Frequently Asked Questions (FAQs)
Q1. Which is more reactive, Haloarenes or Haloalkanes?
Haloarenes are more stable and react less as it is resonance stabilised and has a partial double bond character.
Q2. Why is vinyl chloride is unreactive?
Vinyl chloride (CH2CH2) is stable and unreactive in nucleuphilic suctitution reaction as it is resonance stabilised.
Q3. Why neopentyl bromide undergoes nucleophilic substitution reaction so slowly?
As the bromine is connected to the carbon atom which is surrounded by many alkyl substitutions, it experiences steric hidrance.
