Question
When \(\alpha\)-particles are directed at gold leaf:
1 almost all \(\alpha\)-particles pass through without deflection
2 a few \(\alpha\)-particles are deviated through large angles.
What are the reasons for these effects?
Answer/Explanation
Ans: C
Since the gold nucleus is very small compared to the gold atom, most of the \(\alpha\)-particles miss the nucleus and therefore pass through without deflection.
Some of the \(\alpha\)- particles pass near the nucleus and get deviated through large angles due to Coulomb repulsion because both the alpha particles and the gold nucleus have positive charge.
Question
A nucleus X is radioactive and decays into a nucleus Y.
X and Y are isotopes of the same element.
Which combination of particles could have been emitted during the decay process?
A 1 \(\alpha\) -particle and 1 \(\beta ^{-}\) – particle
B 1 \(\alpha\) -particle and 2 \(\beta ^{-}\) – particles
C 2 \(\alpha\) -particles and 1 \(\beta ^{-}\) – particle
D 2 \(\alpha\) -particles and 2 \(\beta ^{-}\) – particles
Answer/Explanation
Ans: B
Since X and Y are isotopes, therefore the number of protons in the nucleus is same in both X and Y.
(A) When 1 \(\alpha\) -particle and 1 \(\beta ^{-}\) – particle are emitted the number of protons in the nuclues reduces by 1, hence X and Y can not be isotopes.
(B) When 1 \(\alpha\) -particle and 2 \(\beta ^{-}\) – particles are emitted the number of protons in the nucleus remains the same, therefore X and Y can be isotopes.
(C) When 2 \(\alpha\) -particles and 1 \(\beta ^{-}\) – particle are emitted the number of protons in the nucleus reduces by 3, hence X and Y can not be isotopes.
(D) When 2 \(\alpha\) -particles and 2 \(\beta ^{-}\) – particles are emitted, the number of protons in the nucleus reduces by 2, hence X and Y can not be isotopes.
Question
A uranium-238 nucleus, ![]() undergoes a series of nuclear decays to form uranium-234,
undergoes a series of nuclear decays to form uranium-234, ![]()
Which series of decays could give this result?
A emission of four β–particles
B emission of four ϒ-rays
C emission of one a-particle and two β–particles
D emission of two a-particles and eight β–particles
Answer/Explanation
Ans :C
The atomic number of uranium-238 is 92, and its mass number is 238. To decay to uranium-234, it must undergo a series of alpha and beta decays.
A. Emission of four β-particles: This is not possible because beta decay does not change the atomic mass number, so the resulting nucleus would still have a mass number of 238, not 234.
B. Emission of four γ-rays: Gamma rays are high-energy photons emitted during nuclear transitions, but they do not change the atomic number or mass number of the nucleus. Therefore, this option is also not possible.
C. Emission of one α-particle and two β-particles: This is a possible decay series for uranium-238 to form uranium-234. Uranium-238 can undergo alpha decay to form thorium-234, which in turn undergoes beta decay to form protactinium-234, which then undergoes beta decay again to form uranium-234.
D. Emission of two α-particles and eight β-particles: This is not possible because the resulting nucleus would have a mass number of 230, not 234.
Therefore, the correct answer is C: emission of one α-particle and two β-particles.
Question
The figure shows part of a chart of nuclides where neutron number is plotted against proton
number.
An unstable nuclide X decays by emitting an α-particle.
Which nuclide is formed by the decay of nuclide X?
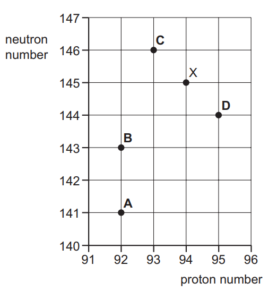
Answer/Explanation
Ans B
Question
A nucleus of uranium,![]() undergoes a series of decays. During the series of decays, two a-particles and one ẞ particle are emitted. As a result, a nucleus of actinium, Ac, is formed.
undergoes a series of decays. During the series of decays, two a-particles and one ẞ particle are emitted. As a result, a nucleus of actinium, Ac, is formed.
What is the correct notation for the nuclide of actinium that is formed?
![]()
Answer/Explanation
Ans:B
