Chapter 5 Nuclear Physics
5.1 The nuclear model of the atom
5.1.1 The atom
CORE
1. Describe the structure of an atom in terms of a positively charged nucleus and negatively charged electrons in orbit around the nucleus
2. Know how atoms may form positive ions by losing electrons or form negative ions by gaining electrons
SUPPLEMENT
3. Describe how the scattering of alpha (α) particles by a sheet of thin metal supports the nuclear model of the atom, by providing evidence for:
(a) a very small nucleus surrounded by mostly empty space
(b) a nucleus containing most of the mass of the atom
(c) a nucleus that is positively charged.
5.1.2 The nucleus
CORE
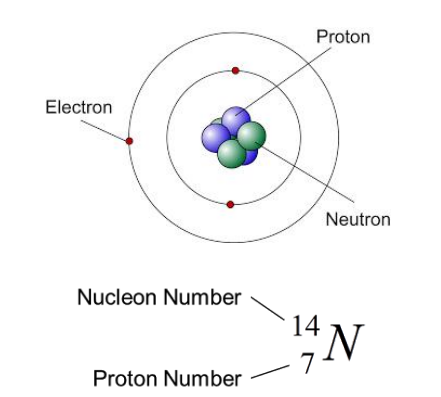
1. Describe the composition of the nucleus in terms of protons and neutrons
2. State the relative charges of protons, neutrons and electrons as +1, 0 and -1 respectively
3. Define the terms proton number (atomic number) Z and nucleon number (mass number) A and be able to calculate the number of neutrons in a nucleus
4 Use the nuclide notation \(^{A}_{Z}X\)
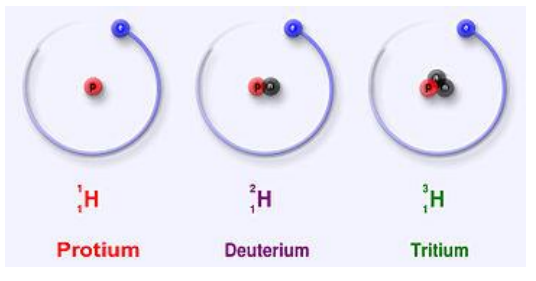
5 Explain what is meant by an isotope and state that an element may have more than one isotope
SUPPLEMENT
6. Describe the processes of nuclear fission and nuclear fusion as the splitting or joining of nuclei, to include the nuclide equation and qualitative description of mass and energy changes without values
7. Know the relationship between the proton number and the relative charge on a nucleus
8. Know the relationship between the nucleon number and the relative mass of a nucleus
• Almost all the mass of an atom is concentrated in the nucleus
• The nucleus consists of protons and neutrons
• Total number of protons and neutrons is called the nucleon number
• Isotopes are atoms of certain elements with the same proton numbers but different nucleon numbers (difference in number of neutrons)
• Isotopes have the same chemical properties but different physical quantities
(e.g., molecular mass, density)
• Protons can be thought of as atomic DNA
• Ions are formed when an atom gains or loses electrons
• Examples of isotopes: Protium, Deuterium, Tritium (isotopes of the hydrogen element)
• Alpha particles are positively charged particles
• Scattering of alpha particles by gold foil refers to the deflection of alpha particles when they collide with the atoms of a gold foil
• The experiment was performed by Ernest Rutherford in 1909 to determine the structure of the atom
• The results of the experiment showed that most of the mass of an atom is concentrated in a small, dense nucleus
• A few alpha particles were deflected at large angles, indicating that they had encountered something massive in the gold foil
• This led Rutherford to propose that the atom consists of a positively charged nucleus surrounded by electrons
• The experiment helped to confirm the atomic model with a central nucleus and electrons in orbit around it.
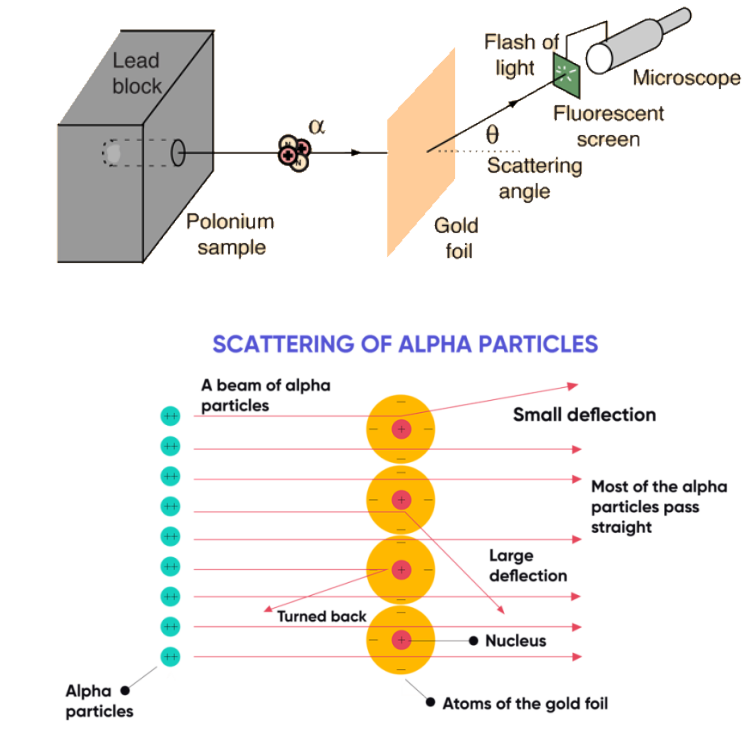
• The 3 findings from the scattering of alpha particles by gold foil experiment:
1) The majority of alpha particles passed through the foil without being deflected.
2) Some alpha particles were slightly deflected, suggesting the existence of empty spaces within the foil.
3) A small number of alpha particles were greatly deflected or completely bounced back, suggesting the presence of dense, positively charged objects within the foil.
• Nuclear fission involves the splitting of a heavy nucleus into two or more smaller nuclei
• The nucleus is typically bombarded by a neutron, causing the nucleus to become unstable
• This instability leads to the nucleus splitting into two or more smaller nuclei
• When the nucleus splits, it also releases a large amount of energy and additional free neutrons
• The free neutrons can then go on to collide with other nuclei, leading to a chain reaction
• This chain reaction can be harnessed to produce nuclear energy or used in nuclear weapons
• Nuclide equation: An example of a nuclear fission reaction is the fission of
uranium-235 (U-235): U-235 + neutron → Ba-141 + Kr- 92 + 3 neutrons
• Nuclear fusion involves the joining of two or more lighter nuclei to form a heavier nucleus
• This requires high temperatures and pressures to overcome the repulsive forces between the positively charged nuclei
• When the nuclei are joined, they form a single, heavier nucleus
• The process releases a large amount of energy, which can also be harnessed to produce energy or used in weapons
• During the process of fusion, a small amount of mass is transformed into a large amount of energy, according to the famous equation \(E=mc^2\). This equation states that energy (E) is equal to mass (m) times the speed of light (c) squared.
In the process of fusion, the total mass of the system decreases, but the total energy released is much greater.
5.2 Radioactivity
5.2.1 Detection of Radioactivity
CORE
1. Know what is meant by background radiation
2. Know the sources that make a significant contribution to background radiation including:
(a) radon gas (in the air)
(b) rocks and buildings
(c) food and drink
(d) cosmic rays
3. Know that ionizing nuclear radiation can be measured using a detector connected to a counter
4. Use count rate measured in counts/s or counts/minute
SUPPLEMENT
5. Use measurements of background radiation to determine a corrected count rate
5.2.2 The three types of nuclear emission
CORE
1. Describe the emission of radiation from a nucleus as spontaneous and random in direction
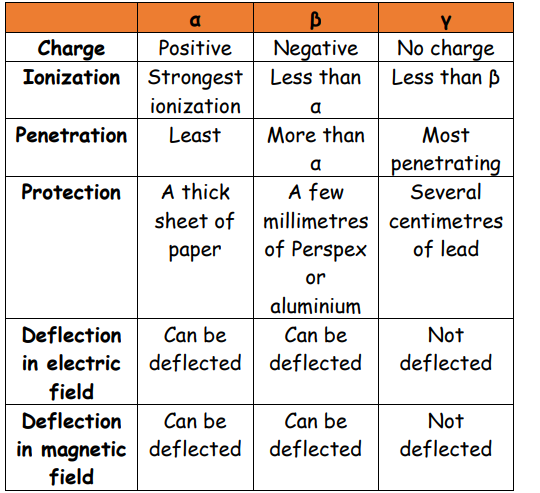
2. Identify alpha (α), beta (β) and gamma (γ) emissions from the nucleus by recalling:
(a) their nature
(b) their relative ionising effects
(c) their relative penetrating abilities (\(β^{+}\) are not included, β-particles will be taken to refer to \(β^{-1}\))
SUPPLEMENT
3. Describe the deflection of α-particles, β-particles and γ-radiation in electric fields and magnetic fields
4. Explain their relative ionising effects with reference to:
(a) kinetic energy
(b) electric charge
• Radioactivity refers to the spontaneous emission of radioactive particles from an unstable nucleus
• The emission is a way for the nucleus to become more stable by reducing its energy
• There are three types of radioactive emission: alpha, beta and gamma
• Alpha emission involves the release of alpha particles, which are helium nuclei
• Beta emission involves the release of beta particles, which are high-energy electrons
• Gamma emission involves the release of gamma rays, which are high-energy photons
• The instability of the nucleus can be due to an imbalance of protons and neutrons, known as isotopes
• The process of radioactivity can result in the nucleus changing into another element through a decay process.
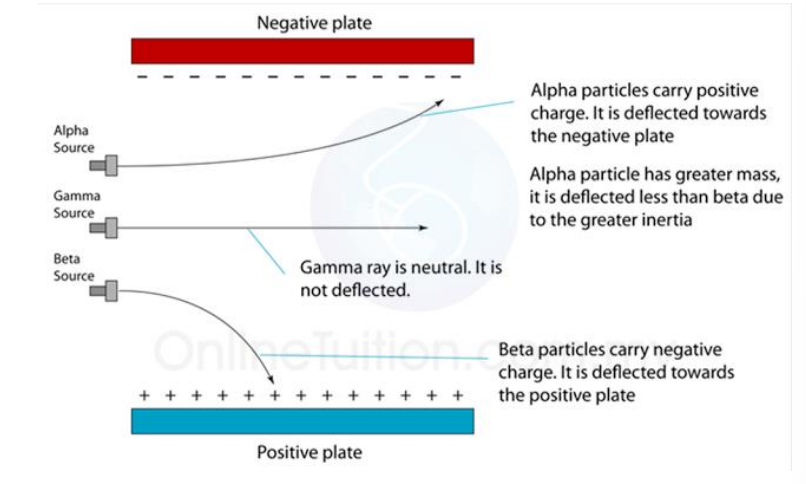
Example problem
Determine which of the following emissions are alpha, beta and gamma emissions
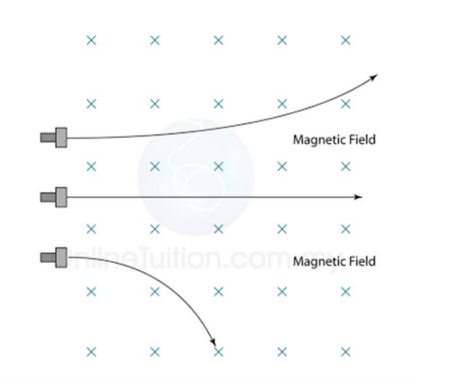
5.2.3 Radioactive decay
CORE
1. Know that radioactive decay is a change in an unstable nucleus that can result in the emission of α-particles or β-particles and/or γ-radiation and know that these changes are spontaneous and random
2. State that during α-decay or β-decay, the nucleus changes to that of a different element
SUPPLEMENT
3. Know that isotopes of an element may be radioactive due to an excess of neutrons in the nucleus and/or the nucleus being too heavy
4. Describe the effect of α-decay, β-decay and γ-emissions on the nucleus, including an increase in stability and a reduction in the number of excess neutrons; the following change in the nucleus occurs during β-emission
neutron → proton + electron
5. Use decay equations, using nuclide notation, to show the emission of α-particles, β-particles and γ-radiation
5.2.4 Half life
CORE
1. Define the half-life of a particular isotope as the time taken for half the nuclei of that isotope in any sample to decay; recall and use this definition in simple calculations, which might involve information in tables or decay curves (calculations will not include background radiation)
SUPPLEMENT
2. Calculate half-life from data or decay curves from which background radiation has not been subtracted
3. Explain how the type of radiation emitted and the half-life of an isotope determine which isotope is used for applications including:
(a) household fire (smoke) alarms
(b) irradiating food to kill bacteria
(c) sterilization of equipment using gamma rays
(d) measuring and controlling thicknesses of materials with the choice of radiations used linked to penetration and absorption
(e) diagnosis and treatment of cancer using gamma rays
• The nucleus of an unstable isotope emits nuclear radiation, including α, β, and γ rays, to become stable
• The process of emitting nuclear radiation is called radioactive decay
• Radioactive decay occurs spontaneously and randomly
• The unstable nucleus before decay is called the parent nuclide
• The stable nucleus produced after decay is called the daughter nuclide
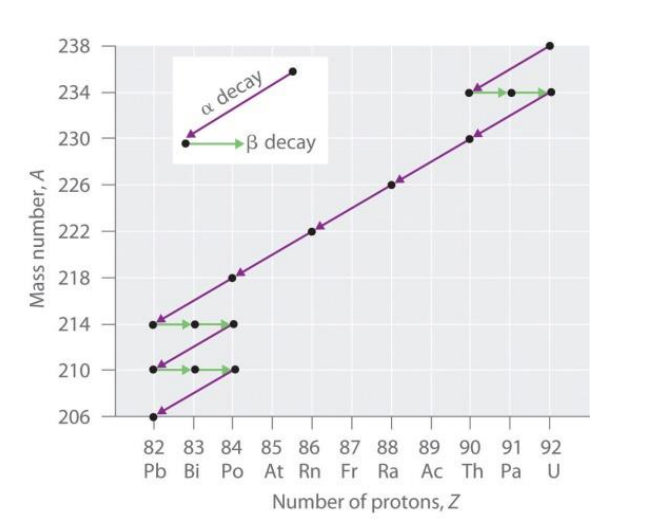
• Alpha decay
\(^{A}_{Z} X \rightarrow ^{A-4}_{Z-2} Y + ^{4}_{2} He\)
1) During an alpha decay, a radioactive atom X decay and emits an alpha particle \((^{4}_{2} He\))
2) Atom X losses 2 neutron and 2 proton and become atom Y.
eg. \(^{238}_{92} U \rightarrow ^{234}_{90} Th + ^{4}_{2} He\)
• Beta decay
\(^{A}_{Z} X \rightarrow ^{A}_{Z+1} Y + {^{0}_{-1} e}\)
1) A beta particle is an electron emitted from a nucleus.
2) The beta particles are very small and move with very high speed.
3) During a beta decay, a radioactive atom X decay and emits a beta particle \(({^{0}_{-1} e}\))
4) One of the neutron is disintegrated to become proton and electron. The electron is emitted out from the nucleus whereas the proton stay in the nucleus
5) Hence, the proton number goes up by 1 while the nucleon number remains unchanged.
eg. \(^{234}_{90} Th \rightarrow ^{234}_{91} Pa + {^{0}_{-1} e}\)
• Gamma Emission
Gamma emission causes no change in nucleon or proton number. This is because gamma ray is an electromagnetic radiation and not a particle.
\(^{A}_{Z} X \rightarrow ^{A}_{Z} Y + γ\)
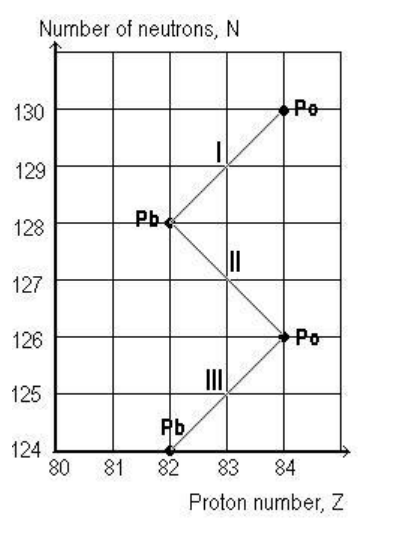
• Unstable nucleus undergoes radioactive decay
• Daughter nucleus may still be unstable
• Daughter nuclide undergoes another radioactive decay
• Process continues until stable nuclide is reached
• This is called series decay
Example problem
State the radioactive decays that the element has gone through.
• Radioactive decay occurs randomly and spontaneously, transforming an unstable nucleus into a more stable one.
• The number of unstable nuclei in a sample decreases with time.
• Half-life is defined as the time taken for the number of unstable nuclei in a sample to reduce to half of its original number.
• Example: Antimony-133 has a half-life of 2.5 minutes.
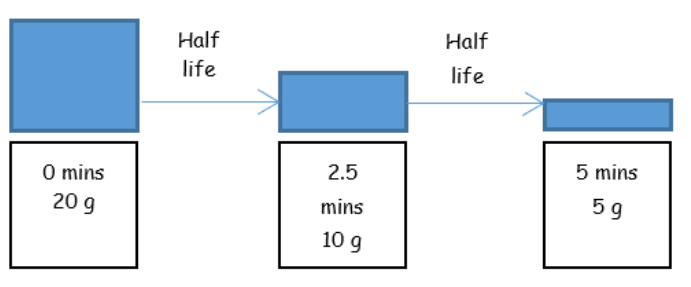
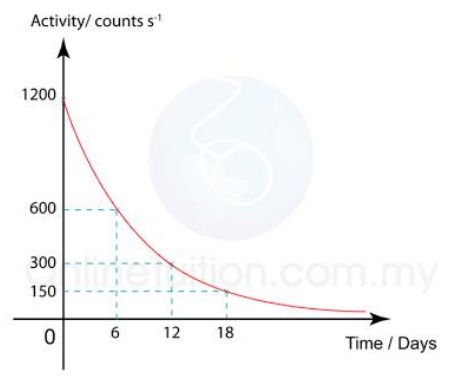
Example problem
The diagram shows the graph of the activity of a radioisotope, X, against time.
What is the half-life of the radioisotope substance?
Radioisotopes have a wide range of applications in various fields. One of the most well known uses of radioisotopes is in archaeology, where Carbon-14 is used for carbon dating. In industry, radioisotopes are used for monitoring the content of food, which helps ensure that the food is safe for consumption. In agriculture, radioisotopes can be used for a variety of purposes, including pest control. By introducing a small amount of radioactive material into the soil, farmers can effectively control the population of pests, reducing damage to crops and increasing yields. Overall, radioisotopes play a significant role in many areas of science and technology, providing valuable tools for research, development, and practical applications.
5.2.5 Safety precautions
CORE
1. State the effects of ionising nuclear radiations on living things, including cell death, mutations and cancer
2. Describe how radioactive materials are moved, used and stored in a safe way
SUPPLEMENT
3. Explain safety precautions for all ionising radiation in terms of reducing exposure time, increasing distance between source and living tissue and using shielding to absorb radiation
• Ionizing nuclear radiation has effects on living things.
• Cell Death: Ionizing radiation has enough energy to damage or kill living cells.
• Mutations: Ionizing radiation can cause changes in the genetic material of living cells, leading to mutations.
• Cancer: Prolonged exposure to ionizing radiation can increase the risk of developing cancer.
• Safe movement, use, and storage of radioactive materials is essential to mitigated the risk of nuclear radiation.
• Proper handling and transportation to minimize exposure to radiation
• Storage in secure, designated areas to prevent contamination
• Use of protective equipment, such as gloves and masks, when handling radioactive materials
• Several safety precautions for handling ionizing radiation are needed.
• Reduce Exposure Time: Minimize the amount of time spent near a source of ionizing radiation.
• Increase Distance: Increase the distance between the source of radiation and living tissue to reduce exposure.
• Use Shielding: Place materials, such as lead or concrete, between the source of radiation and living tissue to absorb radiation and reduce exposure