Question
The Chinese pangolin (Manis pentadactyla) is a critically endangered species that has declined in numbers by 80% since 2000. It inhabits both forest and grassland, where it uses long, powerful claws to open ant and termite nests and ingests the insects using a long, sticky tongue.

(a) (i) State with a reason whether pangolins are autotrophic or heterotrophic.
(ii) Explain what information is needed to find the trophic level of pangolins.
(b) Pangolins are unique among mammals in having evolved scales, which are a recognition feature of reptiles. Explain which features you expect pangolins to have, which would show that they are mammals, not reptiles.
(c) The Chinese pangolin, Manis pentadactyla, has a diploid chromosome number of 40.
(i) State how many chromosomes there would be in gametes of this species.
(ii) Sex is determined in the same way in pangolins as in humans. State how many autosomes there are in somatic cells of M. pentadactyla.
Answer/Explanation
Answer:
(a)
(i) heterotrophic because it feeds on/eats food/other organisms /eats ants/termites/ doesn’t photosynthesise/does not produce its own food;
(ii)
a. what (prey) it eats/feeds on/ stomach content;
b. the trophic level of what (prey) it eats/feeds on/the trophic level of
ants/termites;
c. trophic level is the position an organism occupies in the food chain/web;
(b)
a. hair;
b. mammary glands/milk secretion;
c. alveoli in lungs;
d. lower mandible/jaw consisting of just one bone;
e. giving birth to live young/are placental (apart from duck-billed
platypus/echidna);
f. external ears/ pinna;
g. warm-blooded/endothermic/constant body temperature;
(c)
(i) 20;
(ii) 38
OR
19 pairs;
Question
The diagram shows water molecules as they might be arranged in liquid water and the interactions between them.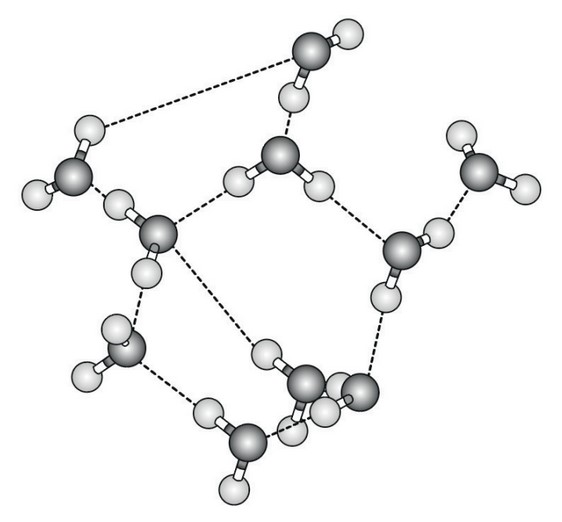
(a) (i) State how many water molecules are shown in the diagram.
(ii) Identify the interactions that are shown between the water molecules.
(b) (i) With reference to the diagram, explain how water in sweat evaporates.
(ii) Outline the reasons for secretion of sweat in humans.
Answer/Explanation
Answer:
(a)
(i) 10;
(ii) hydrogen bonds/H bonds;
(b)
(i) a. heat increases molecular motion/vibration;
b. (hydrogen) bonds break;
c. evaporation is separation of water molecules / water changes from liquid to gas/vapour;
d. heat removed from skin surface/body;
(ii) a. cooling/removing heat/lowering body temperature;
b. to prevent overheating
OR
to help maintain body temperature/for temperature homeostasis/for
thermoregulation
OR
to keep temperature at 37 °C;
Question
a. Draw a labelled diagram to show the fluid mosaic model of the plasma membrane. [4]
b. Outline how neurons generate a resting potential. [4]
c. Hydrogen bonds can exist both within and between molecules in living organisms and have an impact on their structure and function. Explain the importance of hydrogen bonding for living organisms. [7]
▶️Answer/Explanation
Markscheme
a. two correctly orientated layers of phospholipids/phospholipid bilayer shown withheads facing in opposite directions
b. phospholipids shown with two parts labelled hydrophilic/phosphate head AND hydrophobic/hydrocarbon tail
c. protein (any) shown as a globular structure embedded in one/both layers of phospholipid
d. peripheral protein shown as globular structures at the surface of the membrane AND integral protein shown as embedded globular structures
e. glycoprotein shown as embedded globular structure with antenna-like carbohydrateprotruding
OR
carbohydrate shown as branched/antenna-like structure attached either to a protein or to a phospholipid
OR
channel protein(s) shown with a pore passing through it
OR
pump protein shown as a transmembrane globular structure
f. cholesterol shown in between adjacent phospholipids
a. sodium-potassium pump
b. sodium /Na+ out and potassium /K+ in
OR
sodium/Na+ concentration higher outside and potassium/K+ higher inside
c. three Na+ pumped for every two K+ (hence negative inside)
OR
inside of axon holds negative ions/Cl– ions/negatively charged proteins/organic anions (hence negative inside)
d. by active transport / using ATP
e. inside (of axon/neuron) is negative in comparison to outside
OR
electrochemical concentration/charge difference (across the membrane) is the resting potential
f. resting potential is –70 mV
a. cohesion in water/water molecules stick together (due to hydrogen bonds)
b. cohesion helps transport under tension of water/sap in xylem / transpiration stream
c. adhesion between water and cell walls/cellulose/polar molecules
d. adhesion/capillary action helps water to rise in plants/stems/xylem / helps keep leaf walls moist
e. solvent properties (due to hydrogen bonds) with polar/hydrophilic molecules
f. solvent properties exemplified by glucose/other example of a polar solute
g. high latent heat of evaporation / (much) energy required for evaporation so water useful as coolant/for sweating
h. high (specific) heat capacity so water temperature changes less
i. base pairing between bases/nucleotides/strands in DNA by hydrogen bonding
j. base pairing between bases in RNA and DNA for transcription/between codon and anticodon for translation
k. proteins have hydrogen bonding in secondary structure/α helix/β pleated sheet
l. proteins have hydrogen bonding between R groups/in tertiary structure/to maintain conformation
m. habitats because water is liquid due to high boiling point/due to water freezing on the surface
n. habitats on water surface due to surface tension
Question
Plants have developed efficient methods for transport and for synthesis of foods.
a. Outline how the properties of water make it an ideal transport medium in plants. [4]
b. Distinguish between the xylem and phloem of plants. [4]
c. Explain how the light-independent reactions of photosynthesis rely on the light-dependent reactions. [7]
▶️Answer/Explanation
Markscheme
a. polarity of water;
b. hydrogen bonds between water molecules;
c. cohesion between water molecules/water molecules stick together;
d. cohesion allows tensions/low pressures/transpiration pull/movement upward/against gravity;
e. adhesion to cellulose/cell walls generates tensions/pull (in xylem)
OR
adhesion to xylem walls/vessel walls causes capillary rise/upward movement;
f. solvent for many substances / many substances dissolve;
g. liquid at most temperatures experienced by plants / liquid so can flow;
Polarity of water and/or hydrogen bonding can be shown in an annotated diagram.

a. light-dependent reactions produce ATP/reduced NADP;
b. ATP generated by chemiosmosis/by photophosphorylation/by ATP synthase;
c. reduced NADP produced by/using electrons from Photosystem I;
d. RuBP + CO2 to glycerate 3-phosphate (in light independent reactions);
e. glycerate 3-phosphate reduced to triose phosphate (in light independent reactions);
f. ATP/reduced NADP used in the light-independent reactions;
g. reduced NADP provides electrons/hydrogen / to reduce (glycerate 3-phosphate)
OR
reduced NADP used to convert glycerate 3-phosphate to triose phosphate;
h. ATP provides energy (for reduction of glycerate 3-phosphate);
i. ATP needed to regenerate RuBP
j. ATP/reduced NADP run out in darkness
k. Calvin cycle only possible with light/in the day/is indirectly dependent on light;