- IB Style Question Banks with Solution
- IB DP Biology SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Biology HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Biology SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Biology HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
2.2 Water
Essential Idea:
Water is the medium of life
Understandings:
- Water molecules are polar and hydrogen bonds form between them
- Hydrogen bonds and bipolarity explain the cohesive, adhesive, thermal and solvent properties of water
- Substances can be hydrophilic or hydrophobic
Applications:
- Comparison of the thermal properties of water with those of methane
- Use of water as a coolant in sweat
- Modes of transport of glucose, amino acids, cholesterol, fats, oxygen and sodium chloride in blood in relation to their solubility in water
- Describe the structure of an atom (in terms of protons, neutrons and electrons).
- Contrast ion with atom.
- Define anion and cation.
- Contrast covalent, ionic and hydrogen bonds.
- Write the molecular formula for water and draw the atomic structure of the molecule.
- Describe the cause and effect of the polar nature of water.
- Describe where and how water is able to form hydrogen bonds.
- Contrast adhesion with cohesion.
- Outline an example of the cohesive property of water being of benefit to life.
- Outline an example of the adhesive property of water being of benefit to life.
- Explain three thermal properties of water that are useful to living organisms.
- Outline a benefit to life of water’s high specific heat capacity.
- Outline a benefit to life of water’s high latent heat of vaporization.
- Outline a benefit to life of water’s high boiling point.
- Explain why is water such a good solvent.
- List the types of molecules that water will dissolve.
- State that polar and ionic molecules are hydrophilic.
- State that non-polar, non-ionic molecules are hydrophobic.
- Given a diagram of a molecular structure, determine if the molecule is hydrophilic or hydrophobic.
- Compare the physical properties of methane and water.
- Explain why water and methane have different thermal properties based on their molecular structures.
- Explain sweating as a mechanism to cool the body.
- State if the following molecules are hydrophobic or hydrophilic: glucose, amino acids, cholesterol, fats, oxygen, and sodium chloride.
- Outline the mechanism of transport in the blood of the following molecules: glucose, amino acids, cholesterol, fats, oxygen, and sodium chloride.
- State why scientists cannot prove without a doubt that hydrogen bonds exist between water molecules.
Topic 2.2: water
In the Water unit students are introduced to the structure and function of water, as the medium of life. Water has many useful properties, and so it is ubiquitous in life on earth. The useful properties of water arise from its structure.
The unit is planned to take 2 school days
- Water is the medium of life.
- Use theories to explain natural phenomena—the theory that hydrogen bonds form between water molecules explains the properties of water.
- State why scientists cannot prove without a doubt that hydrogen bonds exist between water molecules.
2.2.U.1 Water molecules are polar and hydrogen bonds form between them
- Describe the structure of an atom (in terms of protons, neutrons and electrons).
- Contrast ion with atom.
- Define anion and cation.
- Contrast covalent, ionic and hydrogen bonds.
- Write the molecular formula for water and draw the atomic structure of the molecule.
- Describe the cause and effect of the polar nature of water.
- Describe where and how water is able to form hydrogen bonds.
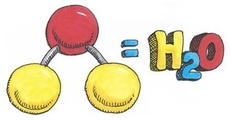
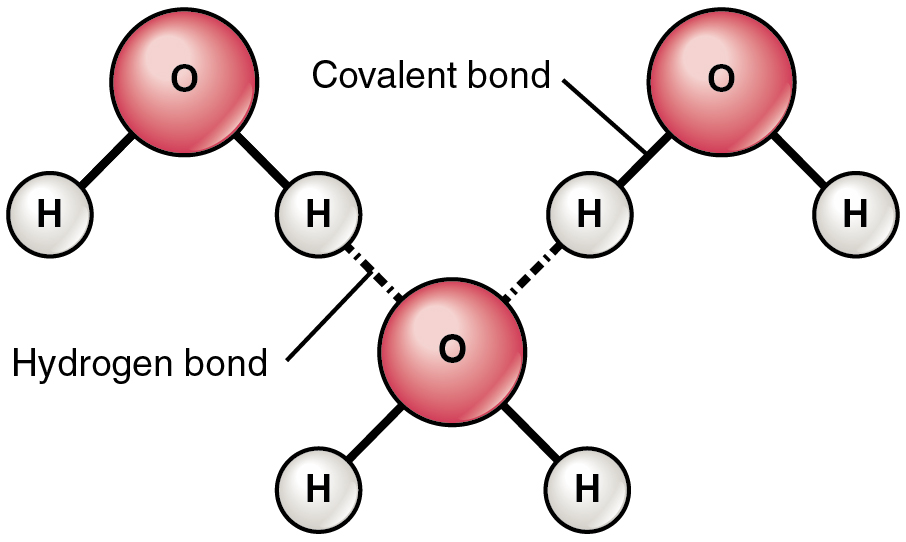
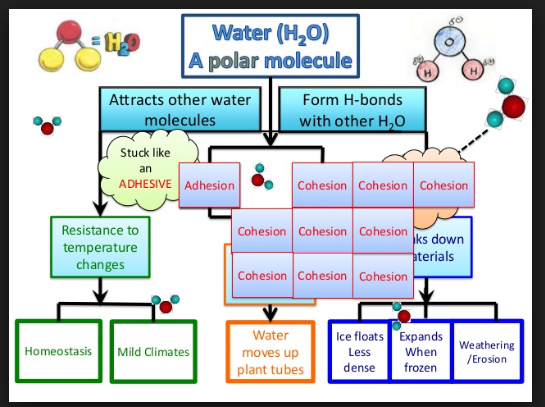
Water molecule formed by hydrogen bonds between oxygen and hydrogen. However sharing of electrons is unequal and are attracted to the oxygen more. Partial negative charge develops on the oxygen and partial positive on the hydrogen. Attraction between water molecules forms a hydrogen bond and forms when a hydrogen atom in one polar molecule is attracted to the slightly negative oxygen atom in the other molecule.
A water molecule consists of an oxygen atom covalently bound to two hydrogen atoms. Since O is more electronegative than H, an unequal sharing of electrons occurs. This creates a polar covalent bond with H having a partial positive charge and O having a partial negative charge.
Water is also bent so the positive charge exists more or less on one side and the negative charge from the O exists on the opposite side. The partial +ve charge is attracted to the partial –ve charge creating an intermolecular attraction between the water molecules called a “Hydrogen bond.”. H-bonds are the strongest of the intermolecular bonding, but is still considered a weak bond; however since there are so many H2O molecules they give water its unique properties and make it essential to life on this planet
2.2.U.2 Hydrogen bonding and dipolarity explain the cohesive, adhesive, thermal and solvent properties of water
- Contrast adhesion with cohesion.
- Outline an example of the cohesive property of water being of benefit to life.
- Outline an example of the adhesive property of water being of benefit to life.
- Explain three thermal properties of water that are useful to living organisms.
- Outline a benefit to life of water’s high specific heat capacity.
- Outline a benefit to life of water’s high latent heat of vaporization.
- Outline a benefit to life of water’s high boiling point.
- Explain why is water such a good solvent.
- List the types of molecules that water will dissolve.
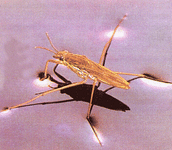
cohesion
- hydrogen bonds between polar water molecules cause them to cohere
- allowing for transpiration in plants moving water against gravity
- surface tension between cohering water molecules
- allowing for animals such as water striders to walk over the surface of ponds even though they are denser than water
adhesion
- hydrogen bonds between polar water molecules and any other charged or ionic substance cause them to cohere
- allowing for transport in an aqueous environment
thermal properties
- hydrogen bonds between polar water molecules cause water to resist change
- high specific heat (energy required to change water temperature)
- high heat of vaporization (energy required to boil water)
- high heat of fusion (loss of energy required to freeze water)
- thus, water produces a stable environment for aquatic organisms
solvent properties
- the polarity of water attracts, or dissolves, any other polar or charged particles by forming hydrogen bonds with them
- proteins, glucose, or ions, such as sodium or calcium are all soluble
- cytoplasm is primarily water, providing a polar medium in which other polar or charged molecules dissolve
- many enzymes are globular proteins that are water soluble so they dissolve in cytoplasm where they control metabolic reactions
2.2.U.3 Substances can be hydrophilic or hydrophobic
- State that polar and ionic molecules are hydrophilic.
- State that non-polar, non-ionic molecules are hydrophobic.
- Given a diagram of a molecular structure, determine if the molecule is hydrophilic or hydrophobic.
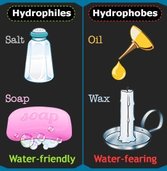
Hydrophilic – A substance that has an affinity for water. These substances can interact or be dissolved by water.(Ex. Like Dissolves Like, detergents, alcohols, salts, cotton,)
Hydrophobic – substances that repel to water. They are non-polar and interact well with other non-polar solvents. An example could be molecules are hydrophobic if they don’t have positive or negative charges
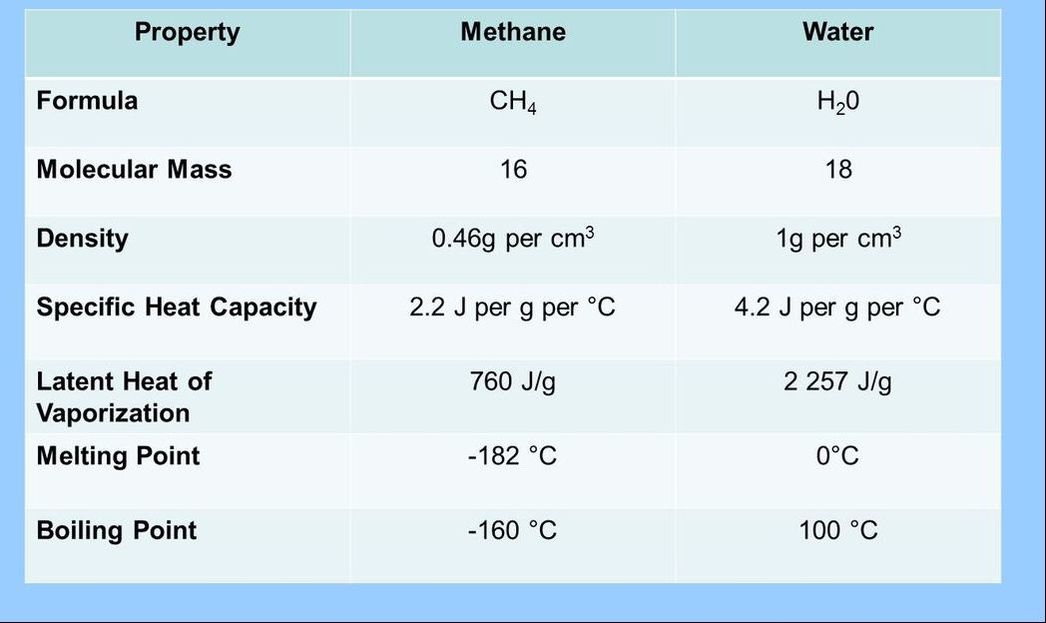
2.2.A.1 Comparison of the thermal properties of water with those of methane.
- Compare and contrast the physical properties of methane and water.
- Explain why water and methane have different thermal properties based on their molecular structures.
2.2.A.2 Use of water as a coolant in sweat
- Explain sweating as a mechanism to cool the body.

2.2.A.3 Modes of transport of glucose, amino acids, cholesterol, fats, oxygen and sodium chloride in blood in relation to their solubility in water.
- State if the following molecules are hydrophobic or hydrophilic: glucose, amino acids, cholesterol, fats, oxygen, and sodium chloride.
- Outline the mechanism of transport in the blood of the following molecules: glucose, amino acids, cholesterol, fats, oxygen, and sodium chloride.
The solvent properties of water mean that many different substances can dissolve in it because of its polarity. This allows substances to be carried in the blood and sap of plants as they dissolve in water. It also makes water a good medium for metabolic reactions.
- Sodium chloride: ions which dissolve and travel through the blood
- Amino acids: Polar and soluble, depending on the R group, soluble enough to be dissolved in blood plasma
- Glucose: polar and freely soluble hence carried through blood plasma
- Oxygen: Non polar but small enough to dissolve in water in very small amounts that is not enough to satisfy aerobic respiration. Haemoglobin present as binding sites for oxygen.
- Fats molecules: Entirely non polar and travel inside lipoprotein complexes which have a single layer of phospholipid also containing proteins.
- Cholesterol: Hydrophobic and are carried via lipoprotein complexes with their hydrophilic region facing outwards
