Question
Double salts are substances with two cations and one anion. A hydrated sulfate containing two cations has this percentage composition.

(a) (i) Draw one Lewis (electron dot) structure of the sulfate ion.
(ii) Calculate the percentage of oxygen present in the double salt.
(iii) Determine the empirical formula of the double salt. Use section 6 of the data booklet.
(iv) The molar mass of the empirical formula is the same as the molar mass of the formula unit. Deduce the formula unit of the hydrated double salt.
(b) 1.20g of the double salt was dissolved in water and an excess of aqueous barium chloride was added, precipitating all the sulfate ions as barium sulfate.
(i) Formulate an ionic equation, including state symbols, for the reaction of barium ions with sulfate ions.
(ii) Calculate the mass of barium sulfate precipitate. Use your answer to part (a)(iii) and section 6 of the data booklet. (If you did not obtain an answer for part (a)(iii),use 400.0g \(mol^{-1}\) as \(M_r\) for the double salt, but this is not the correct value.)
Answer/Explanation
Answer:
(a) (i) 
(ii) «100-(7.09+5.11+16.22+14.91) =» 56.67 «%»
(iii) n(N): 7.09g/14.01g \(mol^{-1}\), n(H): 5.11g/1.01 g \(mol^{-1}\), n(S): 16.22g/32.07 g \(mol^{-1}\),
n(Co): 14.91g/58.93 g \(mol^{-1}\) and n(O): 56.67g/16.00 g \(mol^{-1}\)
OR
n(N): 0.506, n(H): 5.06, n(S): 0.506, n(Co): 0.253 and n(O): 3.54
0.506/0.253, 5.06/0.253, 0.506/0.253, 0.253/0.253, 3.54/0.253
OR
2.00, 20.0, 2.00, 1.00 14.00
\(N_2H_{20}S_2CoO_{14}\)
(iv) \((NH_4)_2Co(SO_4)_2·6H_2O\)
OR
\(Co(NH_4)_2(SO_4)_2·6H_2O\)
(b) (i) ![]()
(ii) 
Question
Analytical and spectroscopic techniques enable chemists to identify and determine structures
of compounds.
(a) An unknown organic compound, X, comprising of only carbon, hydrogen and oxygen was found to contain 48.6% of carbon and 43.2% of oxygen.
(i) Determine the empirical formula.
The mass spectrum of X is shown.
(ii) Identify fragments responsible for the peaks at m/z 74 and 45 using section 28 of the data booklet.
m/z 74:
m/z 45:
The infrared spectrum of X is shown.
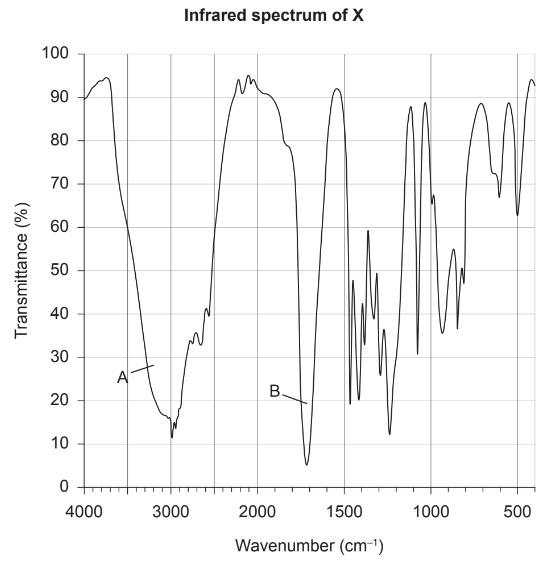
(iii) Identify the bonds making the major contribution to peaks A and B using section 26 of the data booklet.
A:
B:
(iv) Deduce the structural formula of X.
(b) 0.363g of organic liquid Y was vaporized completely at 95.0°C and 100.0kPa. The gas volume was measured to be 81.0\(cm^3\). Determine the molar mass of Y.
Answer/Explanation
Answer:
(a) (i) «n(C) =» 4.05 «mol»
AND
«n(O) =» 2.70 «mol» ü
«% H =» 8.2%
OR
«n(H) =» 8.12 «mol»ü
«empirical formula =» \(C_3H_6O_2\)
(ii) m/z 74:
molecular ion / \(M^+\) / \(C_3H_6O_2^+\)
m/z 45:
\(COOH^+ / C_2H_5O^+\)
(iv) 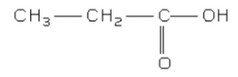
(b) T=368 K AND P = 100.0 kPa AND V = 0.0810 \(dm^3\)
OR
T=368 K AND P = 100 000 Pa AND V = 8.1 x \(10^{−5} m^3\)
n ≪= \(\frac{m}{n} = \frac{0.363 g}{0.00265 mol}≫= 137 «g mol^{−1}\) »