Question
Analytical and spectroscopic techniques enable chemists to identify and determine structures
of compounds.
(a) An unknown organic compound, X, comprising of only carbon, hydrogen and oxygen was found to contain 48.6% of carbon and 43.2% of oxygen.
(i) Determine the empirical formula.
The mass spectrum of X is shown.
(ii) Identify fragments responsible for the peaks at m/z 74 and 45 using section 28 of the data booklet.
m/z 74:
m/z 45:
The infrared spectrum of X is shown.
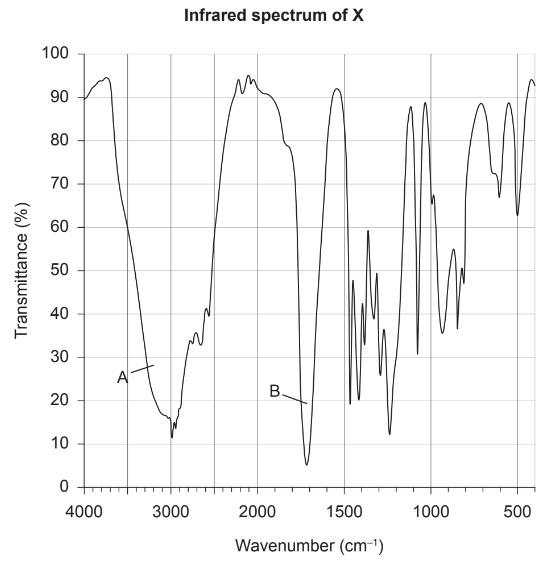
(iii) Identify the bonds making the major contribution to peaks A and B using section 26 of the data booklet.
A:
B:
(iv) Deduce the structural formula of X.
(b) 0.363g of organic liquid Y was vaporized completely at 95.0°C and 100.0kPa. The gas volume was measured to be 81.0\(cm^3\). Determine the molar mass of Y.
Answer/Explanation
Answer:
(a) (i) «n(C) =» 4.05 «mol»
AND
«n(O) =» 2.70 «mol» ü
«% H =» 8.2%
OR
«n(H) =» 8.12 «mol»ü
«empirical formula =» \(C_3H_6O_2\)
(ii) m/z 74:
molecular ion / \(M^+\) / \(C_3H_6O_2^+\)
m/z 45:
\(COOH^+ / C_2H_5O^+\)
(iv) 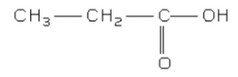
(b) T=368 K AND P = 100.0 kPa AND V = 0.0810 \(dm^3\)
OR
T=368 K AND P = 100 000 Pa AND V = 8.1 x \(10^{−5} m^3\)
n ≪= \(\frac{m}{n} = \frac{0.363 g}{0.00265 mol}≫= 137 «g mol^{−1}\) »