IB PHYSICS SL (Standard level)- 2024 – Practice Questions- All Topics
Topic 5.3 – Electric Cells
Topic 5 Weightage : 8 %
All Questions for Topic 5.3 – Cells , Internal resistance , Secondary cells , Terminal potential difference , Electromotive force (emf)
Question
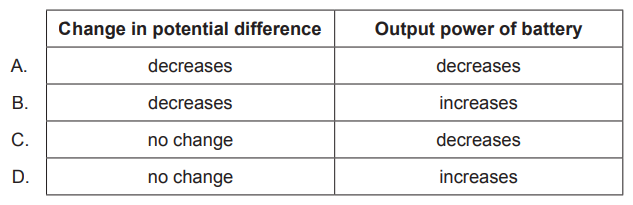
A battery of negligible internal resistance is connected to a lamp. A second identical lamp is
added in series. What is the change in potential difference across the first lamp and what is
the change in the output power of the battery?
▶️Answer/Explanation
Ans: C
Case -1
Let resistance of lamp =R
\( i=\frac{V}{R} \) (V=Voltage of Battery)
Output power of Battery \( =iV=\frac{V^{2}}{R} \)
and, Potential across lamp=V, \( \Delta V=iR \)
\( \Delta V=V_{R}R=V \)
Case- 2 When another identical lamp connected in series ,
\( R_{eq}=2R \) , \( i_{new}=\frac{V}{2R} \)
\( \Delta V=\frac{V}{2R}\times R=\frac{V}{2} \)
Power Output\( =VI=\frac{V^{2}}{2R} \)
Power decreased from \( \frac{V^{2}}{R} \) to \( \frac{V^{2}}{2R} \)
Potentail across lamps changes from V to \( \frac{V}{2} \)
Question
For a real cell in a circuit, the terminal potential difference is at its closest to the emf when
A the internal resistance is much smaller than the load resistance.
B a large current flows in the circuit.
C the cell is not completely discharged.
D the cell is being recharged.
▶️Answer/Explanation
Ans: A
Potential difference and emf are two different quantities whose magnitudes may be equal in certain conditions. The emf is the work done per unit charge by the battery force Fb which is nonelectrostatic in nature. The potential difference originates from the electrostatic field created by the charges accumulated on the terminals of the battery.
The terminal potential difference of a cell is equal to the emf of the cell when:
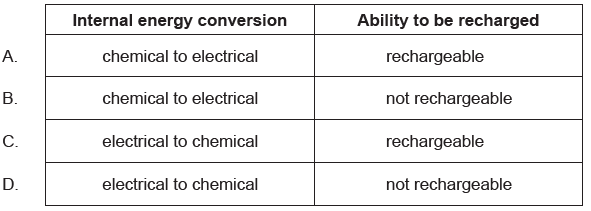
With reference to internal energy conversion and ability to be recharged, what are the characteristics of a primary cell?
▶️Answer/Explanation
Markscheme
B
Primary cell: A primary cell or battery is the one that cannot easily be recharged after one use, and are discarded following discharge. These cell are not chargeable because the electrode reaction occurs only once and after the use over a period of time the batteries become dead and cannot be reused.
Primary cell Properties
1.Chemical reaction is irreversible.
2.Chemical energy is converted into electrical energy.
3.Cannot be recharged
4. Internal resistance is high
5.Can supply weak currents only Light and cheap Example: Simple voltaic cell, Dry cell
A cell is connected in series with a resistor and supplies a current of 4.0 A for a time of 500 s. During this time, 1.5 kJ of energy is dissipated in the cell and 2.5 kJ of energy is dissipated in the resistor.
What is the emf of the cell?
A. 0.50 V
B. 0.75 V
C. 1.5 V
D. 2.0 V
▶️Answer/Explanation
Markscheme
D
Total Energy \( = 1.5kj+2.5 kj\equiv 4kj\)
\(E=V\times I\times t\)
\(4kj=V\times 4\times 500\)
\(V=2 Volt\)
A cell of \({\text{emf }}\varepsilon \) and internal resistance \(r\) delivers current to a small electric motor.
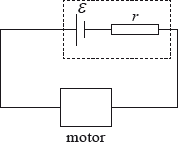
450 C of charge flows through the motor and 9000 J of energy are converted in the motor. 1800 J are dissipated in the cell. The emf of the cell is
A. 4.0 V.
B. 16 V.
C. 20 V.
D. 24 V.
▶️Answer/Explanation
Markscheme
D
\(Energy = V\times I\times t\)
\(i=\frac{c}{t}\)
V.C=Energy
\(v=\frac{(1800+9000)}{450}\rightarrow 24 Volt\)
