8.2 Thermal energy transfer
Essential Idea:
For simplified modelling purposes the Earth can be treated as a black-body radiator and the atmosphere treated as a grey-body.
Understandings:
- Conduction, convection and thermal radiation
- Black-body radiation
- Albedo and emissivity
- The solar constant
- The greenhouse effect
- Energy balance in the Earth surface–atmosphere system
Applications and Skills:
- Sketching and interpreting graphs showing the variation of intensity with wavelength for bodies emitting thermal radiation at different temperatures
- Solving problems involving the Stefan–Boltzmann law and Wien’s displacement law
- Describing the effects of the Earth’s atmosphere on the mean surface temperature
- Solving problems involving albedo, emissivity, solar constant and the Earth’s average temperature
Data booklet reference:
Big Ideas
• Most energy sources can be traced back the sun, our ultimate primary source
• Energy sources must be compared based on many factors including energy density, cost, availability, politics, safety, and environmental impact
• No energy source can be converted to electricity with 100% efficiency
• All energy sources have advantages and drawbacks and it important to understand the complete picture
• Every object with a temperature above 0 K emits thermal radiation
• Radiation intensity is related to separation distance by the inverse square law (similar to force fields)
• The Earth’s climate relies on a delicate thermal energy balance where total energy in equals total energy out
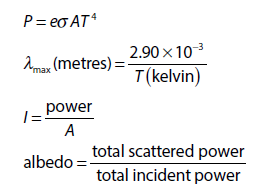
Global Energy Usage
Rank | Energy Source | % |
1 | Oil | 32% |
2 | Coal | 28% |
3 | Natural Gas | 22% |
4 | Biomass | 10% |
5 | Nuclear | 5% |
6 | Hydropower | 2.5% |
Efficiency
Sankey Diagram Rules: Width of the arrow proportional to the amount of energy | 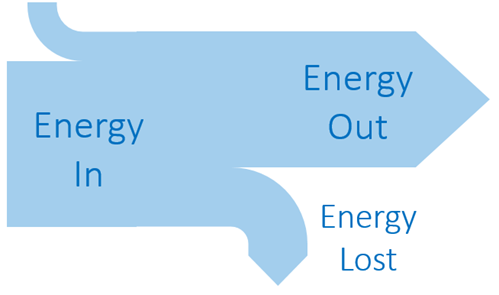 |
Energy Density
Definition | Units | |
Specific Energy | Energy transferred per unit mass | J kg-1 |
Energy Density | Energy transferred per unit volume | J m-3 |
Primary and Secondary Sources
Primary Energy Sources | Secondary Energy Sources |
Energy sources found in the natural environment (fossil fuels, solar, wind, nuclear, hydro, etc.) | Useful transformations of the primary sources (electricity, pumped storage for hydro, etc.) |
Fossil Fuels
Number of years left in global reserves | |
Coal | ~100-150 years |
Oil | ~50 years |
Natural Gas | ~50 years |
Describe the process of Fracking: |
1. Drill hole into shale rock 2. Inject fracking fluid at high pressure to create cracks 3. Extract newly released natural gas 4. Seal fracking fluid in the hole |
Nuclear Power
% of U-235 | |
Uranium Ore | 0.7% |
Fuel-Grade | 3.5% |
Weapons-Grade | 90% |
Why is the concentration of U-235 important? Only U-235 can undergo a fission chain reaction |
What is done with the nuclear waste? Stored on-site in spent fuel pools and/or concrete dry cask storage |
Moderator | Control Rods |
Slows down neutrons to be absorbed by U-235 Made from Water or Graphite (carbon) | Absorbs neutrons to limit number of chain reactions Made from Boron |
Renewable Energy
Variable Symbol | Unit | |
Power | P | W |
Cross-Sectional Area | A | m2 |
Air Density | ρ | kg m-3 |
Air Speed | v | m s-1 |
Data Booklet Equations: |
Photovoltaic Cells | Solar Concentrator | Solar Heating Panel |
Converts solar energy directly into electricity. Useful in solar panels on top of building or solar farms connected to the energy grid | Mirrors focus sunlight onto a central tower. The high thermal energy is converted to steam and runs turbines to produce electricity | Sun’s radiation is absorbed by black pipes that transfer thermal energy to the water flowing through them. Replaces hot water heater. |
Biomass | Coal | Geothermal | Hydropower | Natural Gas | Nuclear | Petroleum | Solar | Wind | |
Renewable | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
Produces CO2 | ✓ | ✓ | ✓ | ✓ |
Thermal Energy Transfer
Conduction | Convection | Radiation |
Energy is transferred through molecular collisions | Energy circulates through the expansion and rising of hot fluids | Energy is transferred through electromagnetic radiation. Can travel through a vacuum |
Emissivity | Black Body Radiation | 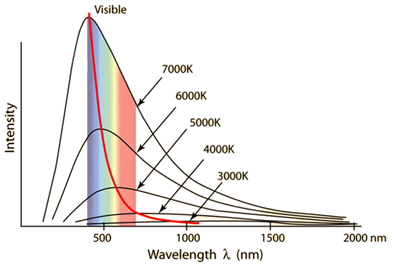 | ||
Sun | ~1 | An idealized object that absorbs all the electromagnetic radiation the falls on it | ||
Earth | ~0.6 | |||
Black-Body | 1 |
Power Emissivity | Variable Symbol | Unit |
Power | P | W |
Emissivity | e | — |
Surface Area | A | m2 |
Temperature | T | K |
Max Wavelength | λmax | m |
Data Booklet Equations: |
Solar Radiation and Climate Change
Intensity | Variable Symbol | Unit | Data Booklet Equations: | |
Intensity | I | W m-2 | ||
Power | P | W | ||
Area | A | m2 |
Greenhouse Gases | Positive Feedback Loop | Negative Feedback Loop | |
Water Vapor (H2O) | Melting ice (decreases albedo) | Cloud formation (increases albedo) | |
Carbon Dioxide (CO2) | Melting permafrost (releases methane) | Increased photosynthesis (uses CO2) | |
Methane (CH4) | Rising ocean temp releases methane | Climate Change leads to renewables |
HEAT TRANSFER
- Conduction
- Convection
- Radiation
CONDUCTION
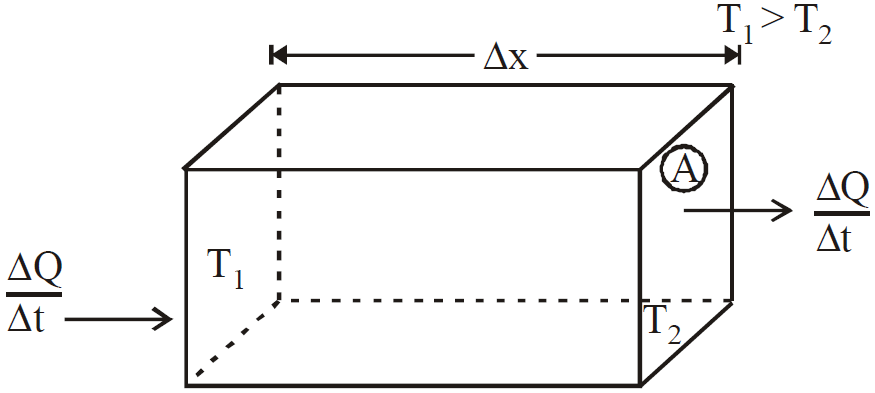
CONVECTION
RADIATION
- The equation
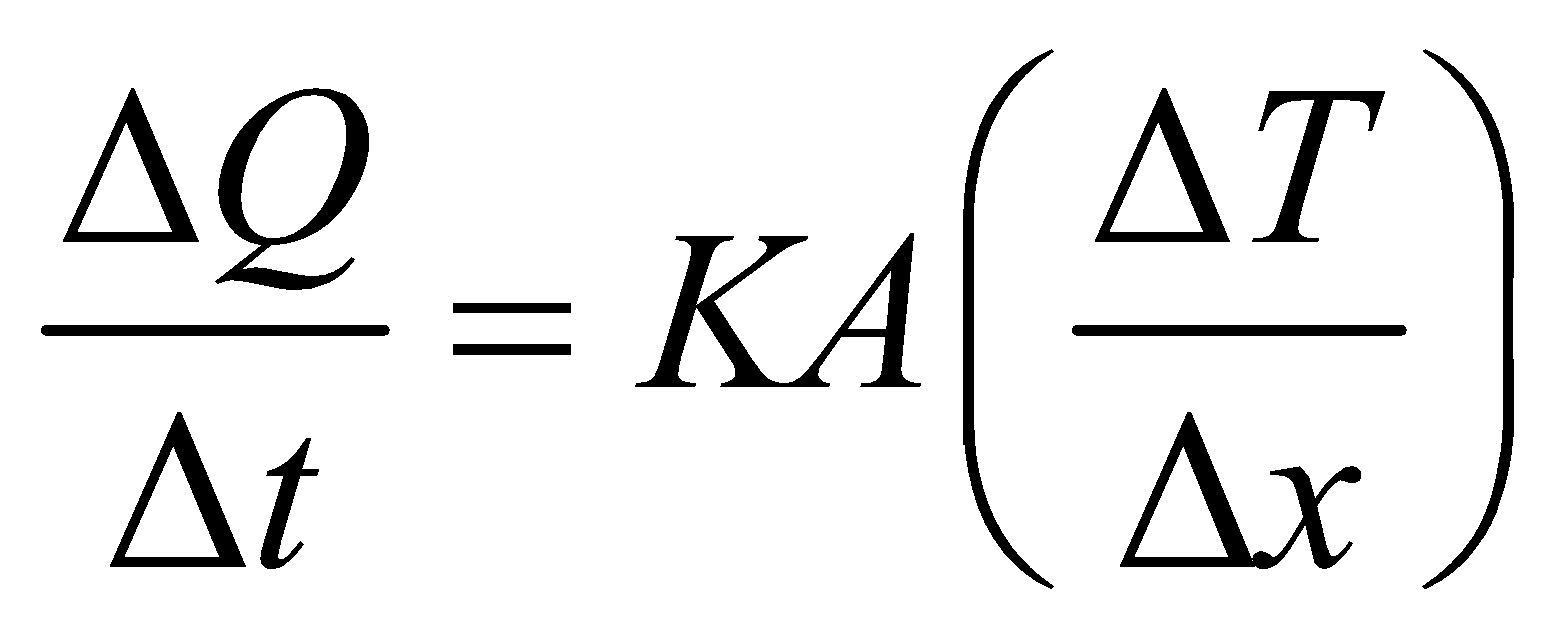 is valid for steady state condition. The condition is said to occur when no part of heat is used up in raising the temperature of any part of cross-section of the solid.
is valid for steady state condition. The condition is said to occur when no part of heat is used up in raising the temperature of any part of cross-section of the solid. - On comparing equation (1) with the following equation used for flowing of charge on account of potential difference.
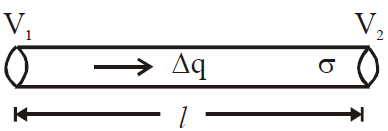
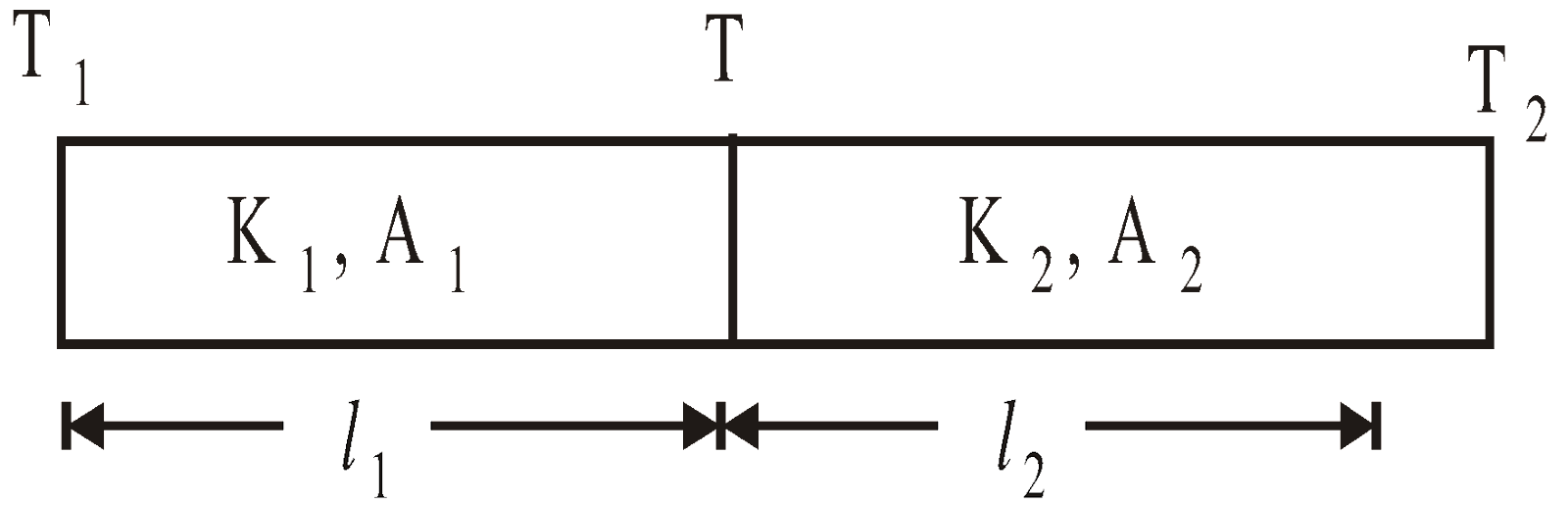
- Series combination of conductors

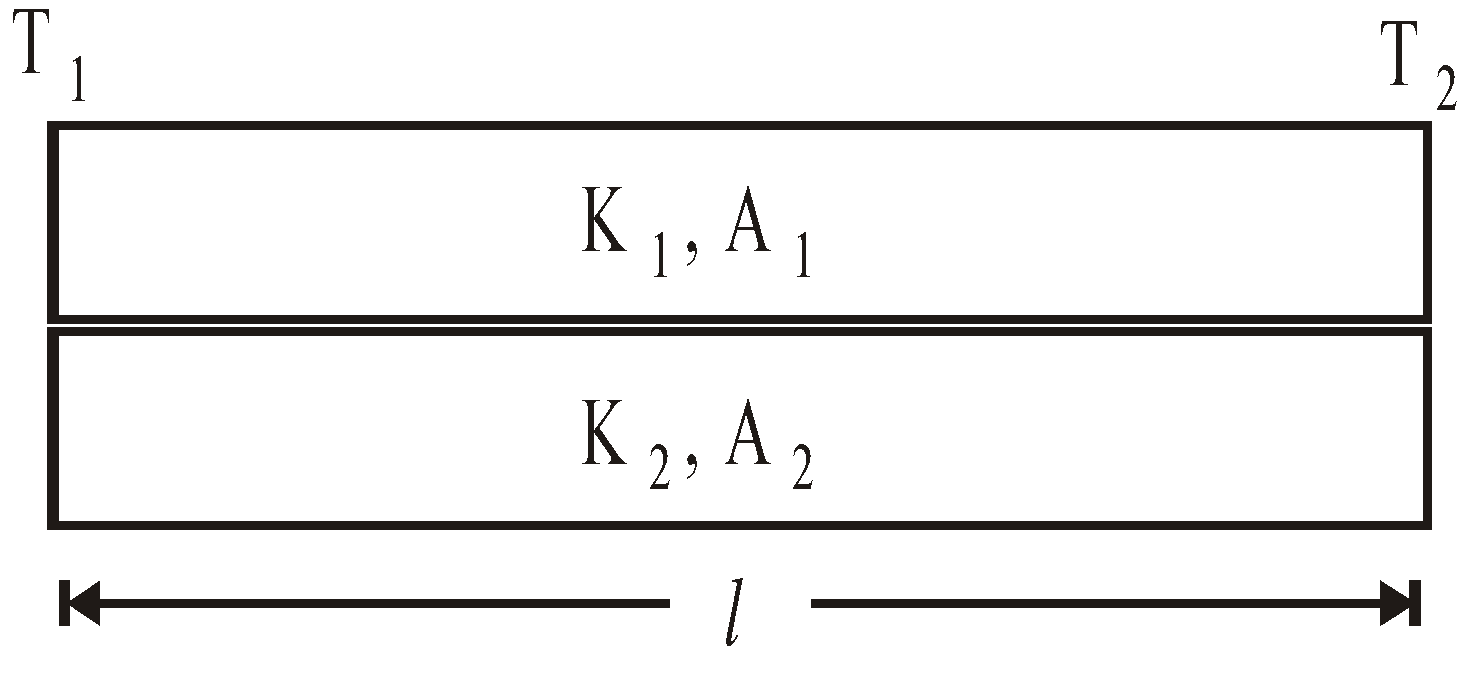
- Parallel combination of conductors
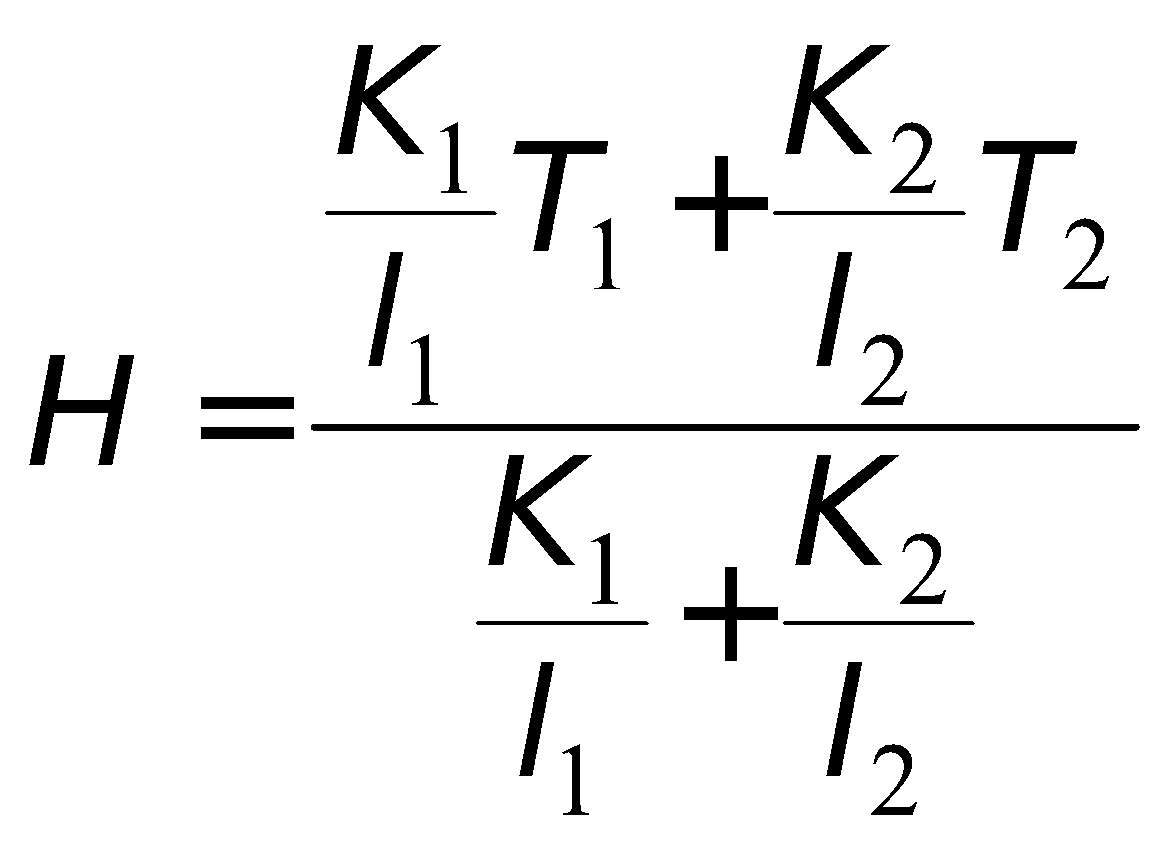
- Davy’s safety lamp is based on the conduction. It is used in mines to know the ignition temperature of gases. Danger of explosion can be avoided.
- Principle of chimney used in a kitchen or a factory is based on the convection.
- Land and sea breezes are due to the convection.
- Temperature of the upper part of the flame is more than the temperature on the sides, because the currents of air carry the heat upwards.
- Radiation can be detected by differential air thermometer, Bolometer, thermopile, etc.
HEAT TRANSFER BY RADIATION AND NEWTON’S LAW OF COOLING
STEFAN’S LAW
σ = 5.67×10–8 Wm–2 K–4.
WIEN’S LAW
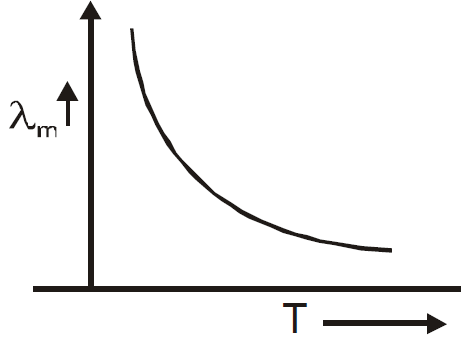
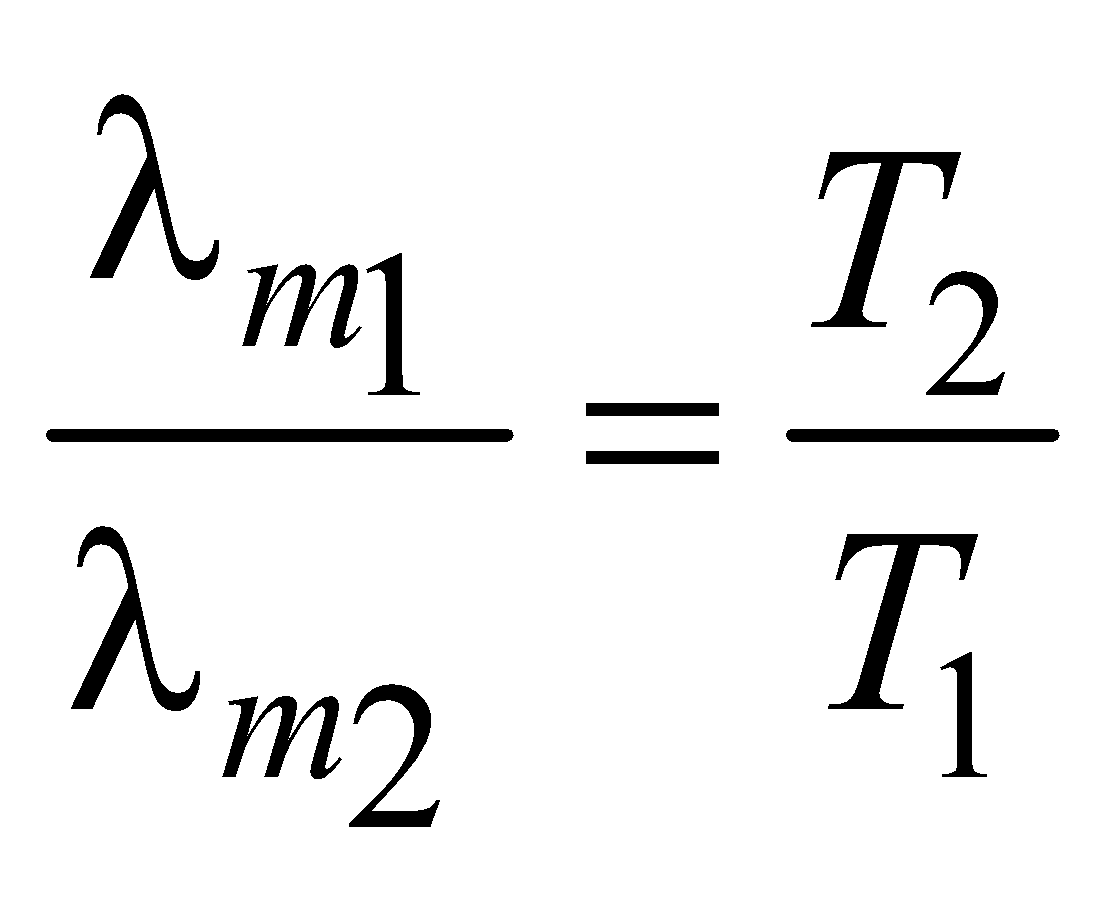
KIRCHOFF’S LAW
- For a perfect black body, A = 1,
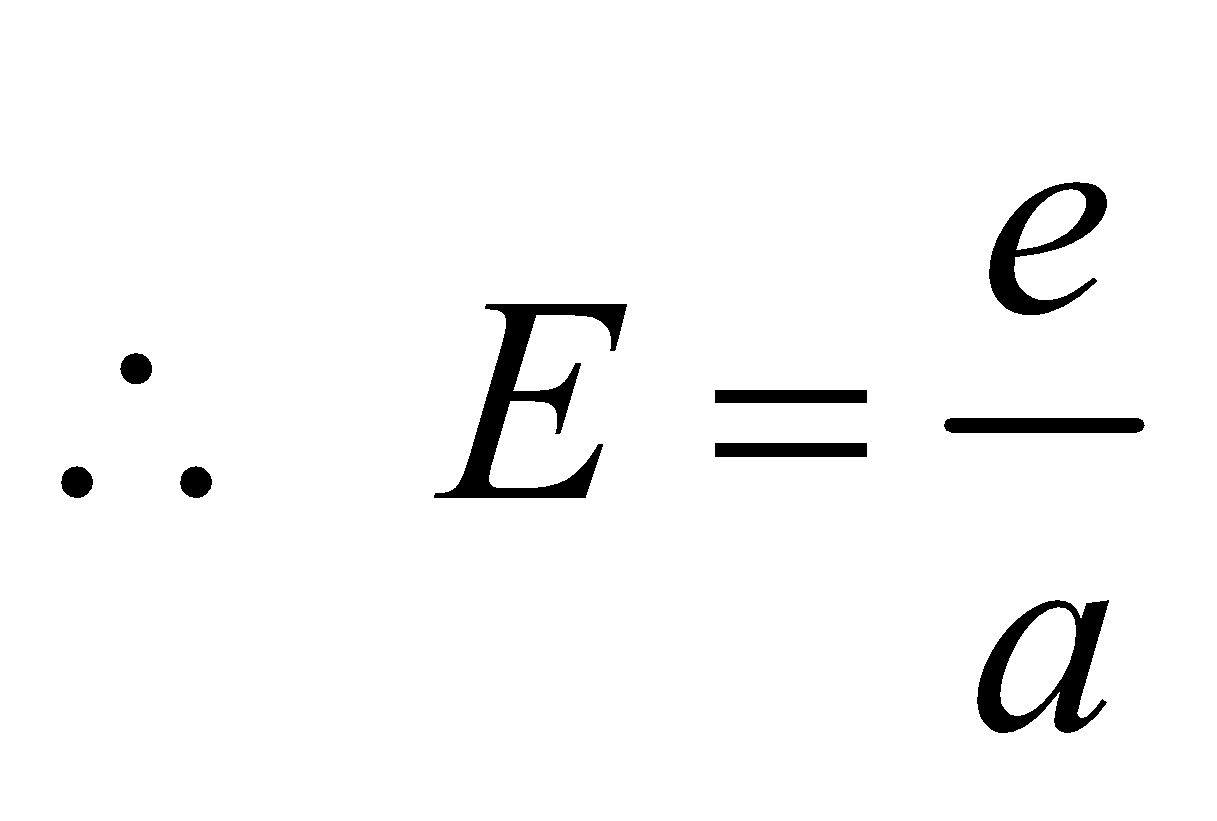
- If emissive and absorptive power are considered for a particular wavelength λ then
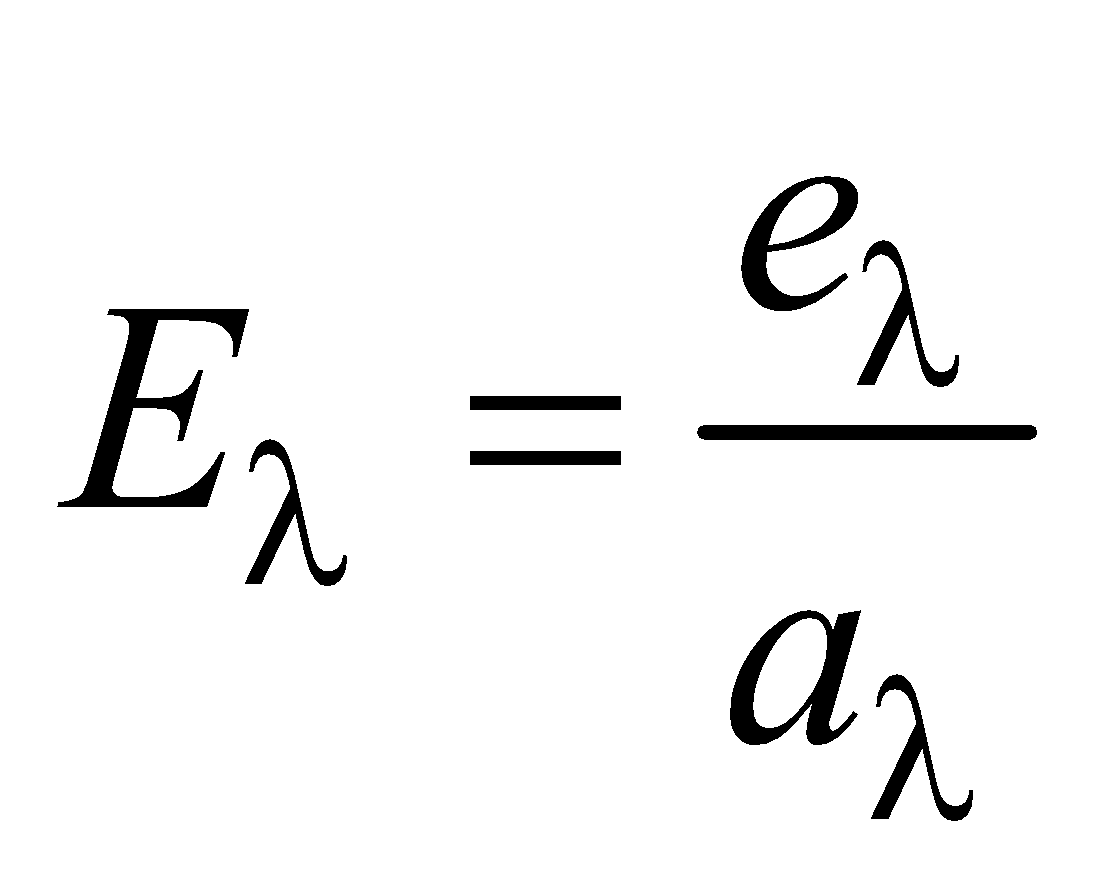
- Since Eλ is constant at a given temperature, according to this law if a surface is good absorber of a particular wavelength then it is also a good emitter of that wavelength.
FRAUNHOFER’S LINE
- Fraunhofer lines are the dark lines in the spectrum of sun and are explained on the basis of Kirchoff’s law. When white light emitted from central core of sun (photosphere) passes through its atmosphere (chromosphere) radiation of those wavelength will be absorbed by the gases present there, which they usually emit (as good emitter is a good absorber) resulting dark lines in the spectrum of sun.
- On the basis of Kirchoff’s law, Fraunhofer identified some of the elements present in the chromosphere. They are hydrogen, helium, sodium, iron, calcium, etc. Fraunhofer had observed about 600 darklines in the spectrum of sun.
NEWTON’S LAW OF COOLING
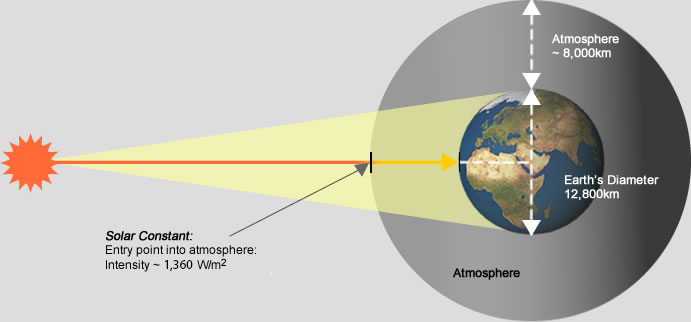
SOLAR CONSTANT
\(P= A\sigma T^4\)
\(\frac{P}{A} = 5.67\times 10^{-8} \times (5800)^4\)
\(=6.42\times 10^7\) Watt
Radius of \(Sun = 6.9 \times 10^8 \; m\)
Hence \( A = 4\pi r^2 = 6 \times 10^{18} \;m^2\)
Hence \( P = 3.9 \times 10^{26}\;W\)
Now distance of Earth from \(Sun = 1.5 \times 10^{11} \; m\)
so the power per unit area at the Earth will be:
\(I = \frac{P}{4\pi r^2}=\frac{3.9 \times 10^{26} }{4\pi(1.5 \times 10^{11})^2}\)
\(=1400 \; W\;m^{-2}\)
- Good absorber is a good emitter.
- Cooking utensils are provided with wooden or ebonite handles, since wood or ebonite is a bad conductor of heat.
- Good conductor of heat are good conductors of electricity, Mica is an exception which being a good conductor of heat is a bad conductor of electricity.
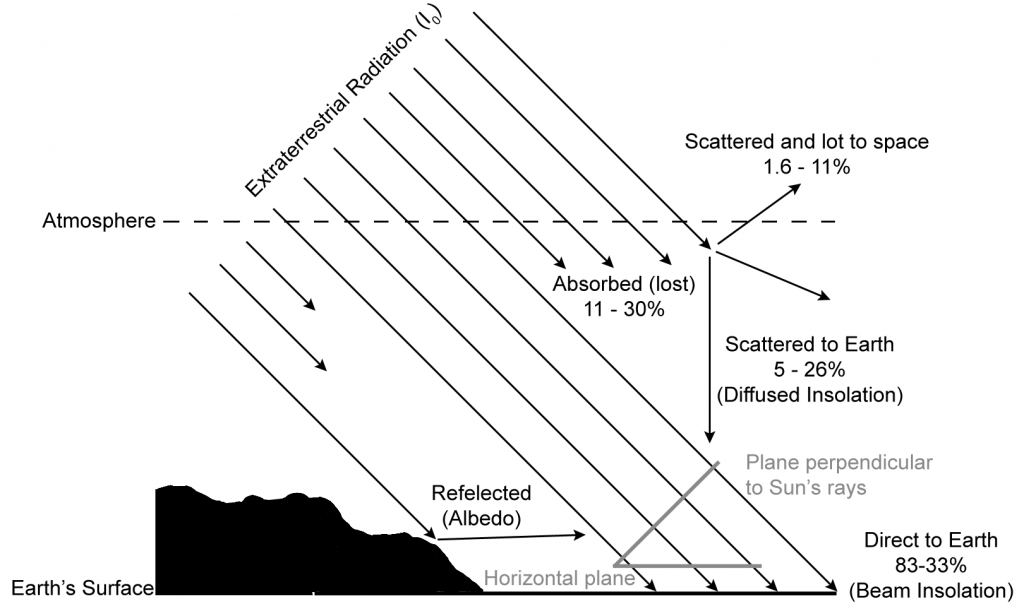
The Atmosphere And Air Mass
The atmosphere scatters and absorbs some of the Sun’s energy that is incident on the Earth’s surface. Scattering of radiation by gaseous molecules (e.g. O2, O3, H2O and CO2), that are a lot smaller than the wavelengths of the radiation, is called Rayleigh scattering. Roughly half of the radiation that is scattered is lost to outer space, the remaining half is directed towards the Earth’s surface from all directions as diffuse radiation. Because of absorption by oxygen and ozone molecules the shortest wavelength that reaches the Earth’s surface is approximately 0.29 µm. Other gas molecules absorbed difference wavelengths as indicated in figure below.
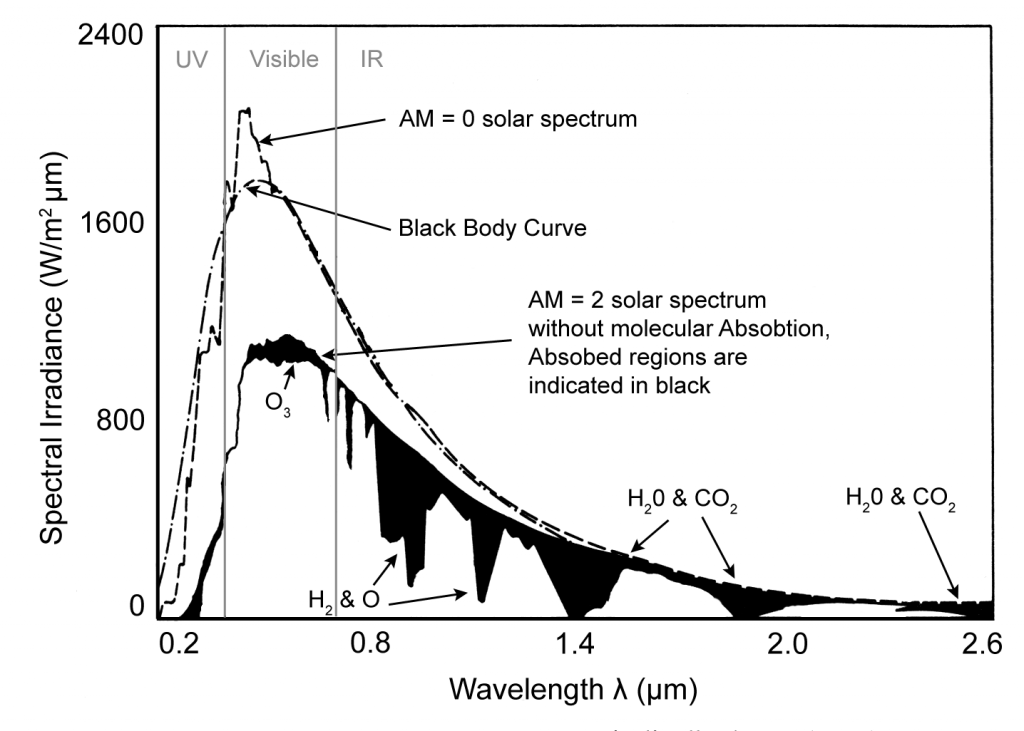
Scattering by dust particles larger than wavelengths of light is called Mie scattering. This process includes both true scattering (where the radiation bounces of the particle) and absorption followed by emission, which heats the particles. The amount of radiation scattered by this process will vary a lot depending on location and the weather blowing particles about. A form of Mie scattering called the Tyndall effect, that preferentially scatters shorter wavelengths is responsible for the sky being blue.
Clouds reflect a lot of radiation and also absorbed a little, the rest is transmitted through. Globally, clouds reflect a lot of radiation and help regulate the surface temperature.