Question:
All elements found in group 1 lose one valence electron when oxidized. What is the major difference between the electrons lost in each of these elements?
▶️Answer/Explanation
Ans: As you go down Group 1, the attraction between the valence electron and the positively charged nucleus decreases. The valence electron is less strongly held and the reactivity of the elements increases down Group 1.
Question:
Give the names, symbols and charges of three cations and three anions that are isoelectronic with the noble gas neon [2,8].
▶️Answer/Explanation
Ans: 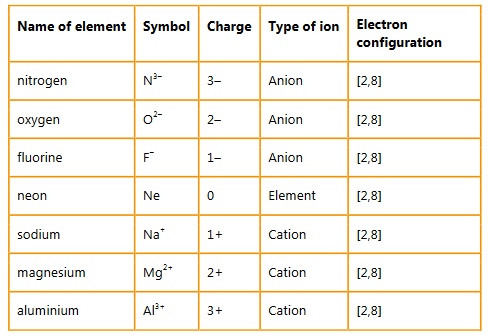
Question:
Copy and complete the following table.
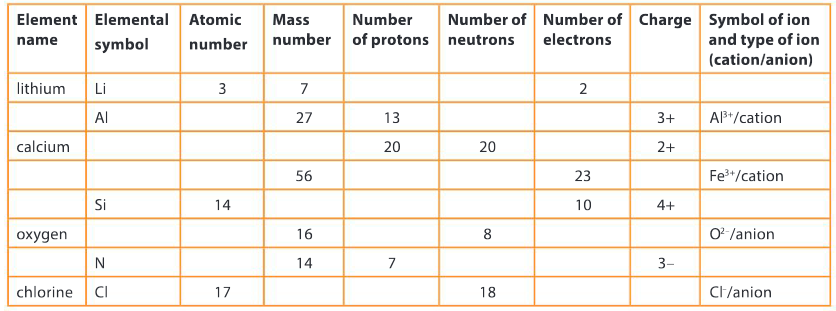
▶️Answer/Explanation
Ans: 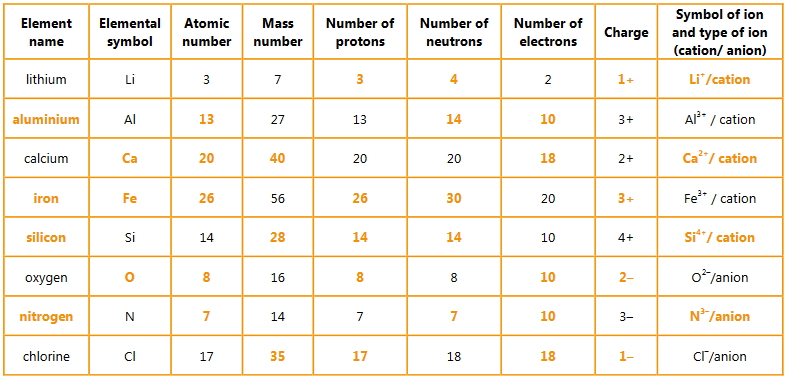
Question:
Do you expect magnesium oxide, MgO, to be more soluble or less soluble than sodium chloride? Explain your reasoning.
▶️Answer/Explanation
Ans: Less soluble; the charges on the ions are greater in MgO (Mg2+ and O2–) than NaCl (Na+ and Cl–). The bonds are therefore stronger in MgO and the ions are less attracted to the water molecules.
Question:
Scientists use three-dimensional visualizations or models to enable a clear understanding of the concept being examined. How do other professions use modelling or simulations to convey meaning?
▶️Answer/Explanation
Ans: Some possible examples of models include:
- Models are used in government departments to plan and visualize transport and infrastructure systems
- Models are used in business and finance industries to predict future economic activity and justify business decisions.
Question:
What did you observe when you placed the seed crystal into the saturated solution?
▶️Answer/Explanation
Ans: When the seed crystal is placed into a saturated solution, fine needle–like crystals begin to appear and eventually fill the beaker.
Question:
Draw conclusions on what is happening to the solubility of the salt as the temperature of the solution decreases. Support your discussion with scientific reasoning.
▶️Answer/Explanation
Ans: As the temperature of the solution decreases, the solubility of the salt decreases. As the temperature decreases, the amount of salt that can dissolve in a given volume of solvent decreases. The addition of the seed crystal provides the stimulus for the excess salt to recrystallize and come out of solution.
Question:
For each of the following pairs of elements, deduce the formula and name.
a) Sodium and fluorine
b) Lithium and oxygen
c) Potassium and nitrogen
d) Calcium and chlorine
e) Magnesium and sulfur
f) Strontium and phosphorus
g) Aluminium and bromine
h) Aluminium and oxygen
i) Aluminium and nitrogen
▶️Answer/Explanation
Ans: a) sodium fluoride, NaF
b) lithium oxide, Li2O
c) potassium nitride, K3N
d) calcium chloride, CaCl2
e) magnesium sulfide, MgS
f) strontium phosphide, Sr3P2
g) aluminium bromide, AlBr3
h) aluminium oxide, Al2O3
i) aluminium nitride, AlN
Question:
For each of the following pairs of elements, deduce the formula and name.
a) Iron(III) and nitrate ion
b) Vanadium(III) and sulfate ion
c) Manganese(VI) and oxide ion (Hint: ionic compounds are the lowest whole number ratio)
d) Copper(I) and carbonate ion
e) Copper(II) and sulfate ion
f) Ammonium ion and nitrite ion
g) Ammonium ion and sulfite ion
h) Nickel(IV) and oxide ion
i) Manganese(VII) and sulfate ion
j) Titanium(II) and hydrogencarbonate ion
▶️Answer/Explanation
Ans: a) iron(III) nitrate, Fe(NO3)3
b) vanadium(III) sulfate, V2(SO4)3
c) manganese(VI) oxide, MnO3
d) copper(I) carbonate, Cu2CO3
e) copper(II) sulfate, CuSO4
f) ammonium nitrite, NH4NO2
g) ammonium sulfite, (NH4)2SO3
h) nickel(IV) oxide, NiO2
i) manganese(VII) sulfate, Mn2(SO4)7
j) titanium(II) hydrogen carbonate, Ti(HCO3)2
Question:
For each of the following elements determine the number of valence electrons and its Lewis symbol.
hydrogen phosphorus fluorine chlorine
boron silicon oxygen
▶️Answer/Explanation
Ans: 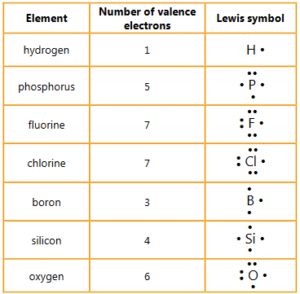
Question:
For each of the following formulae work out the IUPAC names.
a) BF3 b) CH4 c) SO3 d) NF3 e) N2O4
f) Cl2O5 g) PCl3 h) H2O2 i) BCl3 j) OCl2
▶️Answer/Explanation
Ans: a) boron trifluoride
b) methane
c) sulfur trioxide
d) nitrogen trifluoride
e) dinitrogen tetroxide
f) dichlorine pentoxide
g) phosphorus trichloride
h) dihydrogen dioxide
i) boron trichloride
j) oxygen dichloride
Question:
Classify each solution as either a conductor or a non-conductor.
▶️Answer/Explanation
Ans: 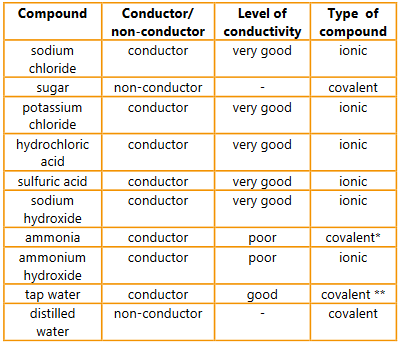
* NH3 + H2O ↔ NH4+ + OH–
** The presence of a wide range of dissolved ions makes tap water a conductor
Question:
For each of the conductors, classify them as poor, good and very good conductors.
▶️Answer/Explanation
Ans: 
* NH3 + H2O ↔ NH4+ + OH–
** The presence of a wide range of dissolved ions makes tap water a conductor
Question:
Find out each compound’s chemical formula and classify it as ionic or covalent. Are the results with respect to conductivity what you predicted given your knowledge of the properties of these compounds?
▶️Answer/Explanation
Ans: 
* NH3 + H2O ↔ NH4+ + OH–
** The presence of a wide range of dissolved ions makes tap water a conductor
Summative assessment
Chemical bonding and its effect on the properties of materials
Question:
Ions formed by metals and non-metals are held together by an electrostatic attraction of unlike charges.
a) Determine the number of valence electrons and the charge formed on each of these elements:
sodium (group 1) calcium (group 2) aluminium (group 13)
nitrogen (group 15) oxygen (group 16) bromine (group 17).
▶️Answer/Explanation
Ans: 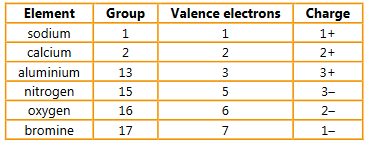
b) Explain how the metallic and non-metallic ions from these groups can combine to form ionic compounds.
▶️Answer/Explanation
Ans: Metals like to lose electrons (oxidation) and form positively charged cations; non–metals like to gain electrons (reduction) and form negatively charged anions; the electrostatic attraction between the oppositely charged ions results in the formation of an ionic bond.
c) Using the elements given in part a) construct formula and give the name of the ions and the resulting ionic compound from the following combinations:
i) group 1 and group 17
ii) group 1 and group 16
iii) group 2 and group 16
iv) group 2 and group 15
v) group 13 and group 16
vi) group 13 and group 17.
▶️Answer/Explanation
Ans: 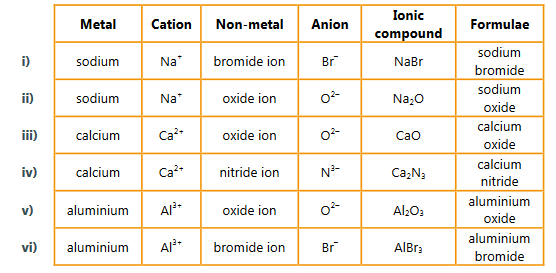
Question:
Use Lewis symbols and structures to explain the formation of the following covalently bonded compounds.
a) Methane
b) Hydrogen bromide
c) Phosphorus pentachloride
d) Oxygen difluoride
e) Carbon dioxide
▶️Answer/Explanation
Ans:
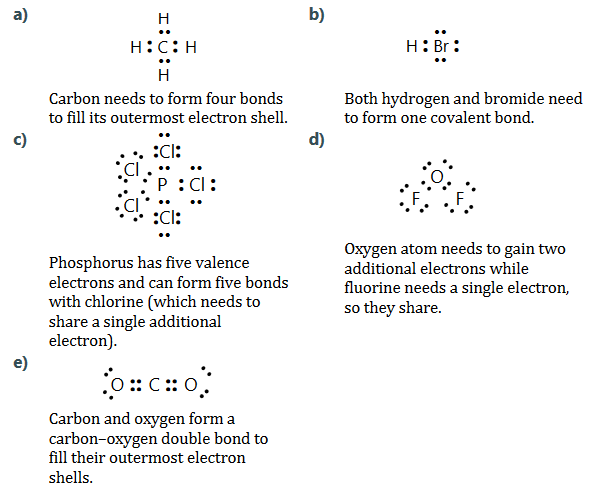
The water molecule has two valence electrons involved in covalent bonding with two hydrogen atoms and two lone pairs of electrons or non-bonding electrons.
In this water molecule, there are electrons involved directly in bonding and the lone pairs of electrons not involved in the formation of the covalent bond.
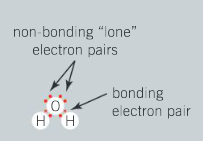
Question:
Consider the compounds in question 2. Can you make the distinction between electrons involved directly in bonding and lone pairs of electrons not required for the formation of the covalent bond? Which molecules in question 2 have lone pairs of electrons and how many?
▶️Answer/Explanation
Ans: Yes; for example, the oxygen atom in OF2 has six valence electrons and only two of the electrons are involved in bonding. This means that on forming two O–F single bonds, oxygen atom has two lone pairs of electrons that are not involved in bonding; bromine in HBr has three lone pairs; chlorine in PCl5 has three lone pairs; oxygen in OF2 has two lone pairs; oxygen in CO2 has two lone pairs.
Question:
Outline the difference between ionic, covalent and metallic bonding focusing on the following:
a) electrical conductivity as a solid
▶️Answer/Explanation
Ans: Metals have delocalized electrons that are able to conduct an electric current; ionic compounds have ions in fixed positions and electrons that are localized therefore they are non–conductors in the solid state; covalent compounds have neither ions nor delocalized electrons and are non–conductors in the solid state.
b) melting point
▶️Answer/Explanation
Ans: Most metals are solids at room temperature and have high melting points due to the strength of the metallic bonding between the metal ions and the sea of delocalized electrons; ionic solids are composed of a large crystalline lattice of oppositely charged ions. They have high melting points as it requires a lot of energy to break the electrostatic attraction between these ions; when simple covalent molecular compounds change state, the weak intermolecular forces between molecules must be broken. This requires little energy and therefore they have low melting points.
c) solubility in water.
▶️Answer/Explanation
Ans: Metals are insoluble in water as they are unable to associate into their ions; ionic compounds are readily soluble in water as they dissociate into their ions and undergo the process of solvation; in general, covalent compounds are insoluble in the polar solvent water.
Question:
Draw a diagram of metallic bonding and use this to explain the following physical properties of a metal:
a) electrical conductivity
▶️Answer/Explanation
Ans: Electrical conductivity: sea of delocalized electrons; positive metal ions in a regular lattice structure.
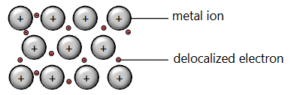
b) malleability
▶️Answer/Explanation
Ans: Malleability: Regular lattice arrangement of the positively charged metal ions; delocalized electrons surrounding the metal ions; no repulsive effects felt when the metal ions are placed under stress and shift positions.
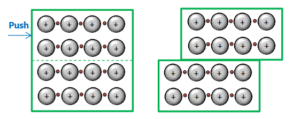
c) ductility (the ability to be drawn into a wire).
▶️Answer/Explanation
Ans: Ductility: When metals are drawn into a wire, the positive metal ions do not repel one another; because they are surrounded by a sea of electrons.

Investigating conductivity
The conductivity of an aqueous solution is dependent on the solute and a number of other factors.
You are provided with the following chemicals and apparatus:
● 0.5 mol dm–3 potassium chloride
● 1.0 mol dm–3 potassium chloride
● 1.5 mol dm–3 potassium chloride
● 1 mol dm–3 calcium dichloride
● 1 mol dm–3 aluminum trichloride
● 1 mol dm–3 hydrochloric acid
● 1 mol dm–3 ethanoic acid (CH3COOH, better known as acetic acid)
● Conductivity probe or equivalent
● Standard laboratory glassware
● Safety glasses

The following hypotheses are all correct. Choose one that you will investigate:
a) An increase in the concentration of a solute will increase the conductivity.
b) The conductivity of a solution is dependent on the number of ions present in a compound.
c) There is a difference in the conductivity of a strong acid when compared to a weak acid.
Support the reason for your choice with scientific reasoning.
Question:
Design an experiment to test your hypothesis. The method should include:
● the independent and dependent variables and other variables being controlled
● recording quantitative and qualitative observations.
▶️Answer/Explanation
Ans: Design should include clear statement of:
- independent and dependent variables
- rationale for the method and practical details, including
- correct names of apparatus and volume
- volumes and concentration of solutions used
- consideration of safety, ethical and environmental issues
- description of the step–by–step methodology for the investigation, including how the variables are controlled
- description of how qualitative observations will be recorded
- identification of quantitative data that will be recorded and the design of data tables to present this information
Marks awarded on a scale from 0 marks for a completely inadequate design to 7 marks for an exemplary design.
How does chemical bonding relate to electrical conductivity?
Following the design of their own investigation on conductivity, students were asked to perform a different investigation on the conductivity of a wide range of substances. The results of this student’s observations have been tabulated below.
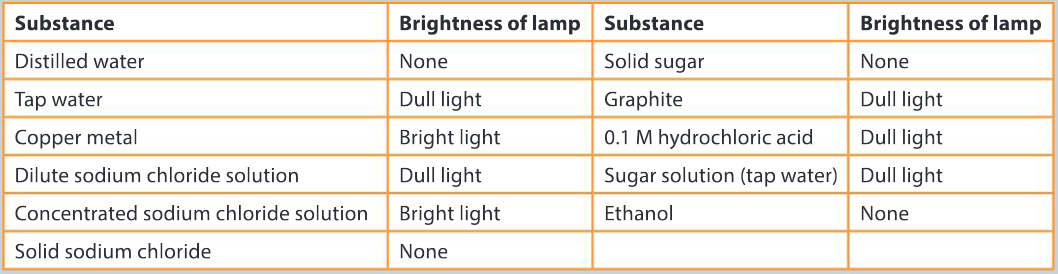
The students tested a range of solids and liquids
Question:
Discuss the differences observed in the level of conductivity in the solids. Your answer should include:
a) comparison of the type of bonding present in each of these solids
▶️Answer/Explanation
Ans: Metallic bonding is present in the element copper; ionic bonding is present in solid sodium chloride; covalent bonding is present in sugar and graphite.
b) an explanation of the features of a solid that enables it to conduct an electric current.
▶️Answer/Explanation
Ans: A solid needs delocalized electrons; that can carry a charge for it to be able to conduct an electric current, a property of metals.
Question:
Examine the results from the testing of distilled and tap water and explain these results using scientific reasoning.
▶️Answer/Explanation
Ans: Distilled water is composed of water molecules and it contains no free ions; tap water is composed of water molecules and dissolved minerals and ions; that are mobile and can conduct an electric current.
Question:
Organize the liquids into groups of good, poor and non-conductors.
a. What features do the good conductors of electricity have in common?
▶️Answer/Explanation
Ans: Good conductors either have delocalized electrons in their bonding as in the case of metals; or can ionize in solution releasing ions that are free to move between electrodes.
b. Suggest how the level of conductivity of the poor conductors could be improved.
▶️Answer/Explanation
Ans: The level of conductivity of a poor conductor can be improved by increasing the number of ions present in solution; and by increasing the temperature of the solution.
Graphene – the wonder material
Question:
List five characteristics, or properties of graphene.
▶️Answer/Explanation
Ans: Graphene has many characteristics or properties, some of them are its strength; lightness; hardness; conductivity; and flexibility.
Question:
Describe three examples of how graphene could be used in your everyday life in the future.
▶️Answer/Explanation
Ans: Individual answers will vary for the applications of graphene.
Question:
The impact of scientific research is far reaching. Explain why the use of graphene in desalination plants could be very significant.
▶️Answer/Explanation
Ans: As graphene can prevent corrosion; and it could be used to make superfine sieves for desalination; reduce the cost of maintenance.
Question:
Utilizing the characteristic properties of graphene, suggest three present technologies that would benefit from the use of graphene in the production process. Give reasons for your answer.
▶️Answer/Explanation
Ans: Some possible technologies that would benefit from the use of graphene in the production process may include:
a) Wearable heart monitors attached to the skin of a patient in the same way you stick an adhesive bandage to your skin – properties include lightness, flexibility and longevity in terms of battery life.
b) Graphene computer processors are at least 30 times faster, lighter and smaller than traditional processors enabling higher powered computers to be more compact than at present.
c) Protective clothing made from graphene is light, strong, and flexible, and could be used by the military, police, or deep sea divers.
Answers for this question will vary as there are a significant number of applications of graphene being developed by science and technology.
