- IB DP Biology 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Biology 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Biology 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Biology 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
A1.1.1 Water as the medium for life
Students should appreciate that the first cells originated in water and that water remains the medium in which most processes of life occur.
In the Water unit students are introduced to the structure and function of water, as the medium of life. Water has many useful properties, and so it is ubiquitous in life on earth. The useful properties of water arise from its structure.
The unit is planned to take 2 school days
- Water is the medium of life.
- Use theories to explain natural phenomena—the theory that hydrogen bonds form between water molecules explains the properties of water.
- State why scientists cannot prove without a doubt that hydrogen bonds exist between water molecules.
List reasons why water is a substance on which life depends.
- The first cells originated in water.
- Water is the “universal solvent” allowing it to dissolve and transport molecules around a body.
- Water is a metabolite in condensation and hydrolysis reactions.
- Water is a temperature buffer in bodies and ecosystems.
- Water maintains biological structures (such as phospholipid bilayer, proteins and DNA).

A1.1.2—Hydrogen bonds as a consequence of the polar covalent bonds within water molecules
Water molecules are polar and hydrogen bonds form between them
- Describe the structure of an atom (in terms of protons, neutrons and electrons).
- Contrast ion with atom.
- Define anion and cation.
- Contrast covalent, ionic and hydrogen bonds.
- Write the molecular formula for water and draw the atomic structure of the molecule.
- Describe the cause and effect of the polar nature of water.
- Describe where and how water is able to form hydrogen bonds.
Hydrogen bonds are attractions of electrostatic force caused by the difference in charge between slightly positive hydrogen ions and other, slightly negative ions. In the case of water, hydrogen bonds form between neighboring hydrogen and oxygen atoms of adjacent water molecules. The attraction between individual water molecules creates a bond known as a hydrogen bond
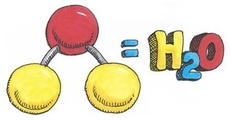
Water molecule formed by hydrogen bonds between oxygen and hydrogen. However sharing of electrons is unequal and are attracted to the oxygen more. Partial negative charge develops on the oxygen and partial positive on the hydrogen. Attraction between water molecules forms a hydrogen bond and forms when a hydrogen atom in one polar molecule is attracted to the slightly negative oxygen atom in the other molecule.
A water molecule consists of an oxygen atom covalently bound to two hydrogen atoms. Since O is more electronegative than H, an unequal sharing of electrons occurs. This creates a polar covalent bond with H having a partial positive charge and O having a partial negative charge.
Water is also bent so the positive charge exists more or less on one side and the negative charge from the O exists on the opposite side. The partial +ve charge is attracted to the partial –ve charge creating an intermolecular attraction between the water molecules called a “Hydrogen bond.”. H-bonds are the strongest of the intermolecular bonding, but is still considered a weak bond; however since there are so many H2O molecules they give water its unique properties and make it essential to life on this planet
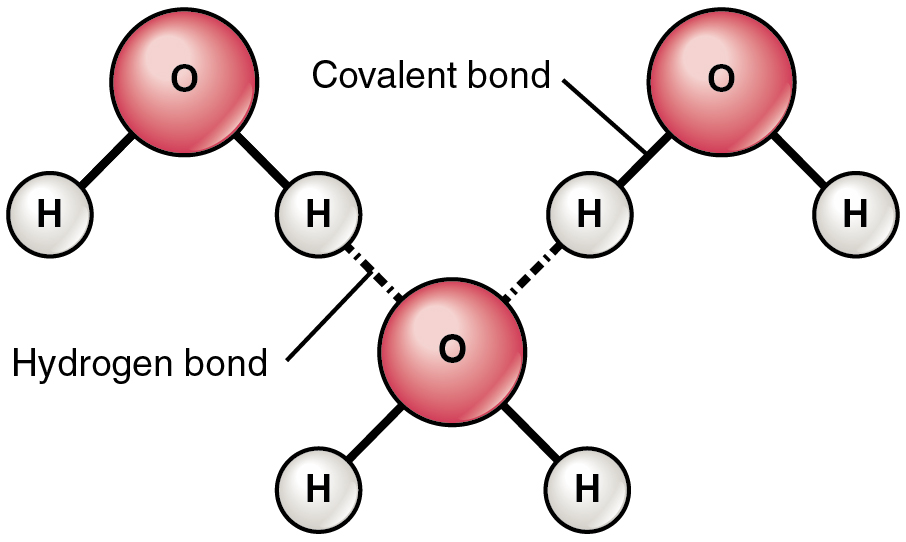
State the location of the polar covalent bond within a water molecule.
- There are two polar covalent bonds within a water molecule; one between the oxygen atom and each hydrogen atom

A1.1.3—Cohesion of water molecules due to hydrogen bonding and consequences for organisms
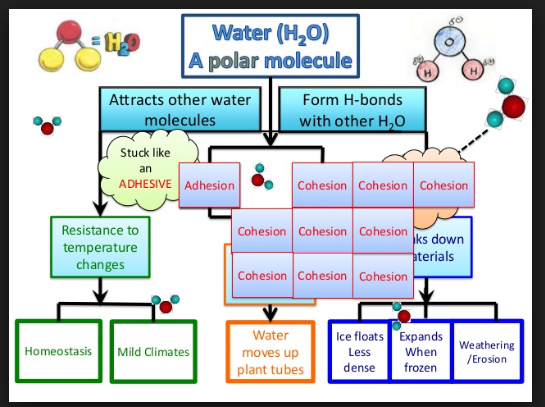
Hydrogen bonding and dipolarity explain the cohesive, adhesive, thermal and solvent properties of water
- Contrast adhesion with cohesion.
- Outline an example of the cohesive property of water being of benefit to life.
- Outline an example of the adhesive property of water being of benefit to life.
- Explain three thermal properties of water that are useful to living organisms.
- Outline a benefit to life of water’s high specific heat capacity.
- Outline a benefit to life of water’s high latent heat of vaporization.
- Outline a benefit to life of water’s high boiling point.
- Explain why is water such a good solvent.
- List the types of molecules that water will dissolve.
cohesion
- hydrogen bonds between polar water molecules cause them to cohere
- allowing for transpiration in plants moving water against gravity
- surface tension between cohering water molecules
- allowing for animals such as water striders to walk over the surface of ponds even though they are denser than water
Water has an amazing ability to adhere (stick) to itself and to other substances. The property of cohesion describes the ability of water molecules to be attracted to other water molecules, which allows water to be a “sticky” liquid.
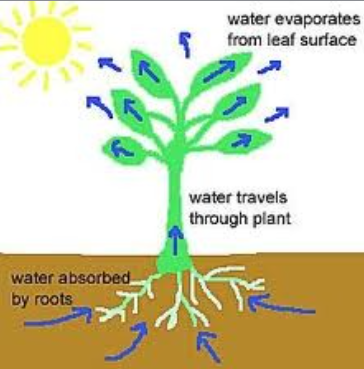
The cohesive property of water and the structure of the xylem vessels allow transport under tension.
- Describe structure of xylem.
- Outline how xylem is able to maintain rigidity even under low pressure or mechanical disturbance.
- Outline polarity of water molecule.
- Define cohesion.
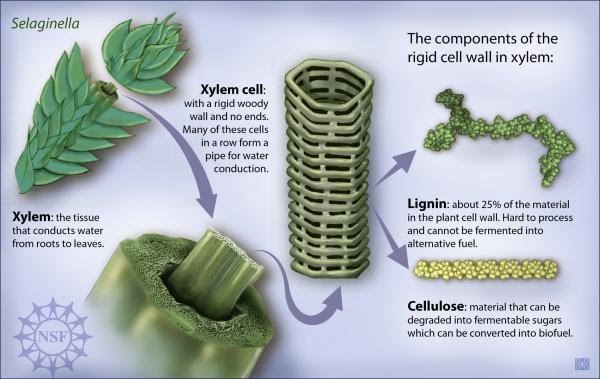
Xylem vessels are transport tissue found in vascular plants composed of a number of different types of cell, including long, continuous, thin, usually dead cells known as tracheids and vessel elements. Xylem vessels have a cell wall strengthened with lignin wich thickens and makes them stronger. Since atmospheric pressure is greater than the pressure inside the xylem vessels, the ridged structure prevent them from collapsing.
The xylem is responsible for the transport of water and soluble mineral nutrients from the roots to the different parts of the plants (stems then leaves) that use water. This also allows minerals absorbed from the soil to be transported through the xylem to the leaves
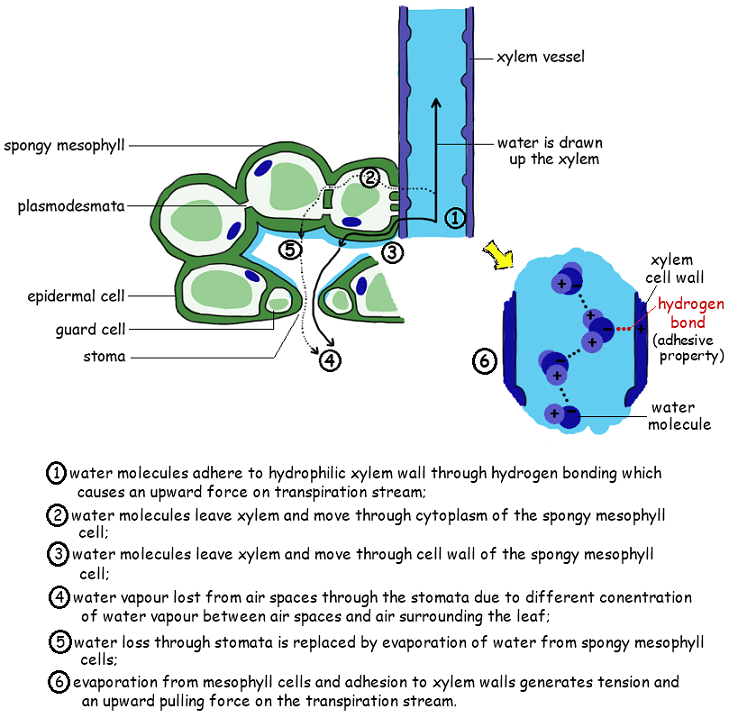
Water molecules cling together by hydrogen bonds between the molecules. This is know as cohesive forces and helps water to to be pulled through the plant. Along with the adhesive forces of water molecules to the xylem walls, a strong tension force is created within the xylem vessel. The cohesive property of the water provides an unbroken column of water in the xylem throughout the plant. Failure to have the cohesive property would stop all flow of water through the xylem vessels.
Define cohesion.
Cohesion is the property of water in which it makes hydrogen bonds with itself, causing water molecules to stick together

Describe how water moves through the xylem of a vascular plant.
- Transpiration (evaporation) occurs through stomata of a leaf. As transpiration occurs, it creates negative pressure
- The tension created by transpiration “pulls” water in the plant xylem, drawing the water upward
- Cohesion pulls up water molecules in a chain as the top-most water is pulled up and out of the stomata.
Outline the cause of surface tension.
The molecules on the surface are more attracted other molecules of the liquid than to molecules in the surrounding air. The net effect is an inward force that causes water to behave as if its surface were covered with a stretched elastic membrane.
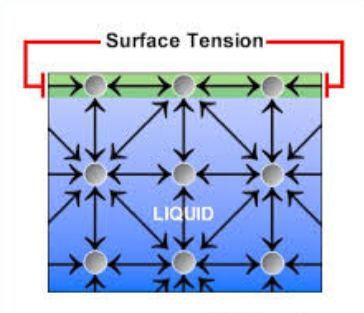
State a benefit to living things that results from surface tension.
Surface tension allows organisms like water striders to “walk on water” and provides a stable environment for other organisms that live on or near the surface of water. To break through the surface of the water, enough force must be applied to break many hydrogen bonds simultaneously.

A1.1.4—Adhesion of water to materials that are polar or charged and impacts for organisms
adhesion
- hydrogen bonds between polar water molecules and any other charged or ionic substance cause them to cohere
- allowing for transport in an aqueous environment
These cohesive forces are related to water’s property of adhesion, or the attraction between water molecules and other molecules. This attraction is sometimes stronger than water’s cohesive forces, especially when the water is exposed to charged surfaces such as those found on the inside of thin glass tubes known as capillary tubes. Adhesion is observed when water “climbs” up the tube placed in a glass of water: notice that the water appears to be higher on the sides of the tube than in the middle. This is because the water molecules are attracted to the charged glass walls of the capillary more than they are to each other and therefore adhere to it. This type of adhesion is called capillary action.
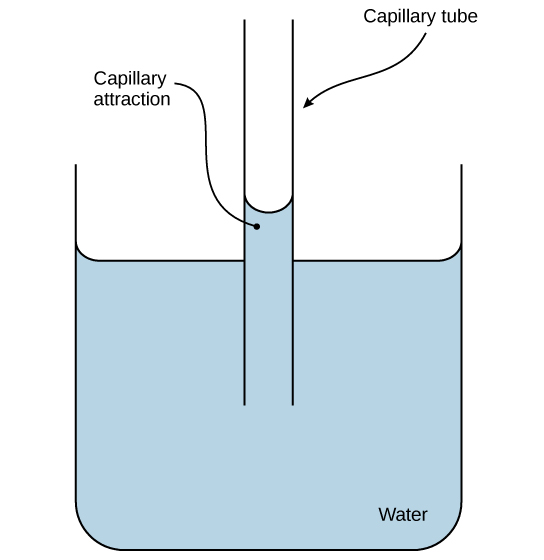
The adhesive property of water and evaporation generate tension forces in leaf cell walls.
- Explain the decrease in pressure and transpiration-pull that results from evaporation of water from the leaf.
- State the transpiration is a passive processes.
adhesion:
- the cell walls of xylem vessels are charged, attracting water molecules
- the adhesive attraction of water to xylem vessel walls moves them up the stem against gravity
- adhesion is important when sap starts to rise in plants that were leafless through the winter
- adhesion also helps prevent the column of water-filled xylem vessels from breaking
Define adhesion.
The attraction of water to other polar or charged molecules is called adhesion.
Outline the cause of capillary action.
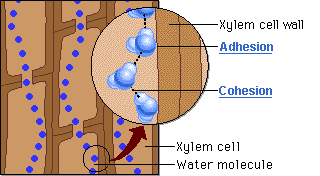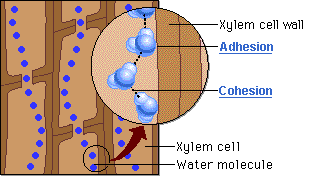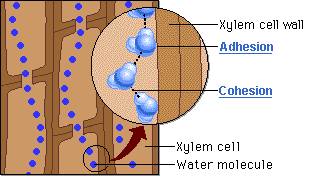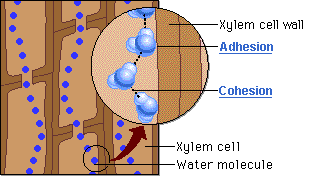
Capillary action is the movement of water in through a narrow space, often in opposition to external forces like gravity. Adhesion of water to the walls of a vessel will cause an upward force on the liquid

Describe capillary action in plant tissue.
Capillary action helps bring water from the roots all the way up to the branches and leaves. Adhesion of water to the xylem walls will cause an upward force on the water.
A1.1.5—Solvent properties of water linked to its role as a medium for metabolism and for transport in plants and animals
solvent properties
- the polarity of water attracts, or dissolves, any other polar or charged particles by forming hydrogen bonds with them
- proteins, glucose, or ions, such as sodium or calcium are all soluble
- cytoplasm is primarily water, providing a polar medium in which other polar or charged molecules dissolve
- many enzymes are globular proteins that are water soluble so they dissolve in cytoplasm where they control metabolic reactions
Substances can be hydrophilic or hydrophobic
- State that polar and ionic molecules are hydrophilic.
- State that non-polar, non-ionic molecules are hydrophobic.
- Given a diagram of a molecular structure, determine if the molecule is hydrophilic or hydrophobic.
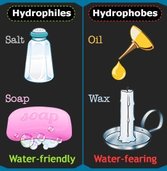
Hydrophilic – A substance that has an affinity for water. These substances can interact or be dissolved by water.(Ex. Like Dissolves Like, detergents, alcohols, salts, cotton,)
Hydrophobic – substances that repel to water. They are non-polar and interact well with other non-polar solvents. An example could be molecules are hydrophobic if they don’t have positive or negative charges
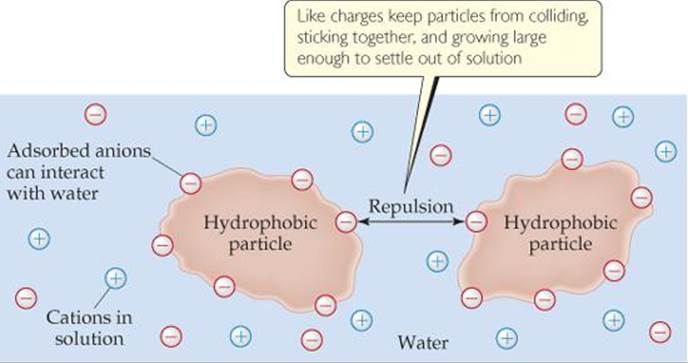
Explain why water is able to dissolve charged molecules.
Water is electrostatically attracted to ions.
The slight positive charge (δ+) of of water hydrogen atom is attracted to the negative charge of an anion .
The slight negative charge (δ-) of of water oxygen atom is attracted to the positive charge of a cation.
Outline the solvation of hydrophilic and hydrophobic substances.
Hydrophobic means water fearing. Because they are not polar or charged, hydrophobic molecules will not dissolve in water. They are insoluble.
Hydrophilic means water loving. Because they are polar or charged, hydrophilic molecules will dissolve in water. They are soluble.
Outline the role of water as a medium for metabolism.
Cytosol is the liquid part of the cytoplasm, a structure common to all cells. It is composed of about 80 percent water and also contains dissolved salts, fatty acids, sugars, amino acids, and proteins such as enzymes. These substances must be dissolved in water in order to carry out the metabolic processes required to keep the cell alive
Describe the role of water as a medium for transport in vascular plants.
Water is used to transport molecules through the body of vascular plants.
Dissolved mineral ions are transported in the xylem from roots to leaves
Dissolved sugars produced in photosynthesis are transported in the phloem from source to sink

Describe the role of water as a medium for transport in animal blood.
Water is used to transport molecules through the body within the blood of animals. Blood plasma transports Salt ions, Amino acids, Proteins, Glucose, Waste products of metabolism and A small amount of dissolved gasses.

A1.1.6—Physical properties of water and the consequences for animals in aquatic habitats

Differences between the physical properties of air and water have major consequences for organisms living in different habitats.
Consider the ringed seal (a mammal) and the Arctic or black-throated loon (a bird).

An adult black-throated loon (Gavia arctica) has a length of about 65 cm and a wing span of 120 cm

A ringed seal (Pusa hispida) peeks its head above water in the Laptev Sea near Russia. A ringed seal rarely grows longer than 150 cm
- They are both of moderate size and have overlapping habitats.
- Both spend time on land rearing their young and in the water foraging for food.
- However, the Arctic loon ties while the ringed seal spends far more time submerged in the water.
The energy requirements for movement in these habitats vary due to differences in buoyancy and viscosity of the medium. Air is less dense, so it provides far less buoyant force. The loon must expend more energy to stay alot than the ringed seal floating in water. Water is more viscous, so the seal must use more energy to move through it. There is about 800 times more drag on a body moving through water than through air at the same velocity.
Outline the cause and effect of buoyancy.
Cause:
an upward force applied to an object that is immersed in a fluid.
Effect:
If the buoyant force of the fluid is greater than the object’s weight, the object will float.
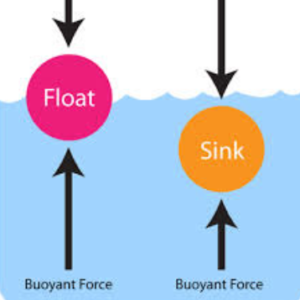
Outline the cause and effect of viscosity.
Cause:
Viscosity is due to the amount of friction the molecules of a liquid experience as they flow over each other.
Effect:
A thick fluid is more viscous and a thin fluid is less viscous.
Compare viscosity of air to water to blood.
Air < Water < Blood
Molecules in air have little friction as they flow over each other, so air is not viscous.
Molecules in water can form hydrogen bonds with each other, increasing the friction and viscosity.
Cells and dissolved solutes in blood cause even more friction as the blood flows, increasing viscosity.
Define thermal conductivity.
Thermal conductivity is a measure of a material’s ability to move heat across a temperature gradient.
thermal properties
- hydrogen bonds between polar water molecules cause water to resist change
- high specific heat (energy required to change water temperature)
- high heat of vaporization (energy required to boil water)
- high heat of fusion (loss of energy required to freeze water)
- thus, water produces a stable environment for aquatic organisms
Outline a consequence to life of the thermal conductivity of air and water.
Marine mammals are exposed to both air and water. The animal will lose more body heat to the environment when in water than in air because water rapidly absorbs and removes heat from the body. As a result, these animals have large layers of blubber which insulate their body heat sources (muscles) from the environmental. Fat is less conductive of thermal energy than water, so the animal is able to retain more body heat than it would be able to without the blubber.
State two benefits to life of the high specific heat capacity of water.
As a result of its high specific heat capacity, water heats up or cools down very slowly. This provides for a stable internal environment and habitat of living things.
Describe how the black-throated loon (Gavia arctica) interacts with the physical properties of water in their habitat.
Buoyancy in water allows the bird to stay afloat without expending a lot of energy, however when flying through air the bird must expend energy to stay aloft. Air is not viscous, so the loon can easily move through it when flying. The loon doesn’t lose as much body heat to the air because air has low thermal conductivity. However, because the air has a low specific heat, its temperature changes as rapidly.
Describe how the ringed seal (Pusa hispida) interacts with the physical properties of water in their habitat.
Buoyancy in water allows the seal to stay afloat without expending a lot of energy. However, the water is viscous, so the seal has adaptations for streamlining as it swims through it. Water has a greater thermal conductivity than air, so the seal needs to insulate itself with blubber to maintain body temperatures. However, because the water has a high specific heat, the temperature of the water does not change as rapidly as the air around it, providing habitat stability for the seal
Additional higher level
A1.1.7—Extraplanetary origin of water on Earth and reasons for its retention
Explain the hypothesis that asteroids are responsible for the origin of water on Earth.
Numerous planetary bodies, including asteroids and comets, containing large amounts of water. asteroids up to a few hundred kilometers across seem the most likely sources of most of Earth’s water. Studies of rocks that date to soon after the formation of Earth suggest that water may have begun to exist on Earth as early as 4.4 billion year ago.

State two reasons why water was retained on early Earth.
- The distance of the Earth from the Sun keeps temperature on Earth below that needed to vaporize water.
- Earth’s gravity keeps water from escaping the planet.

Explain why the presence of water is considered fundamental to the search for extraterrestrial life.
Water is essential for all life because functions as a solvent, allowing key chemical reactions to take place. Other characteristics that make it a good habitat for life are its heat conduction, surface tension, and its high specific heat. In fact, water is so vital to life that the presence of water is precedent to the search for extraterrestrial life.
Define “Goldilocks zone” in relation to the search for extraterrestrial life.
Astrobiologists search for extraterrestrial objects that fall in a Goldilocks Zone, meaning it is just the right distance from a star for water to remain at least periodically in liquid form on the surface