- IB DP Biology 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Biology 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Biology 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Biology 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
D1.2 Protein synthesis
Introduction to molecular biology of the cell
The Central Dogma of Molecular Biology
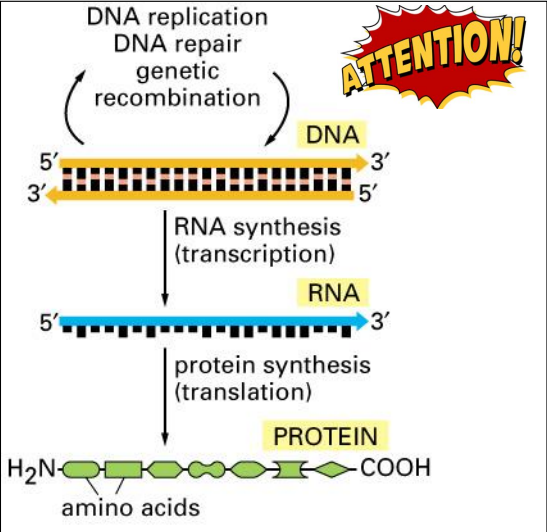
DNA Replication
independent
Synthesis of DNA
One DNA double helix …
…two DNA double helices
Transcription
Step one of protein synthesis
Synthesis of mRNA
A region of DNA is used to…
…produce one mRNA
Translation
Step two of protein synthesis
Synthesis of protein
One mRNA is used to…
…produce one protein
DNA contains the genetic information used to produce proteins
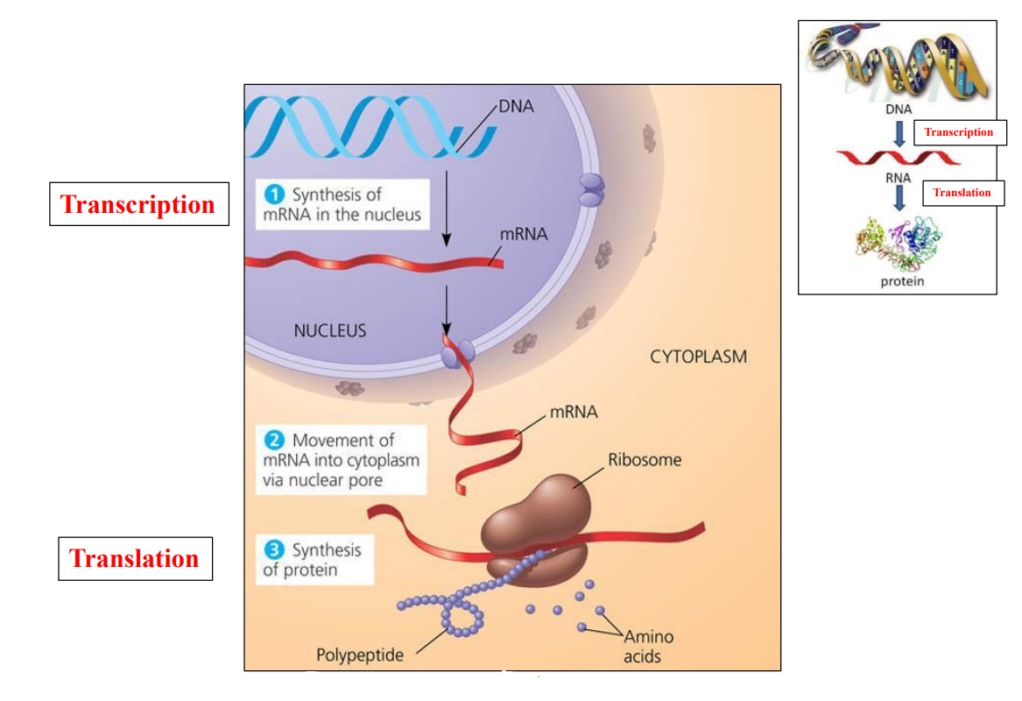
Prokaryotes
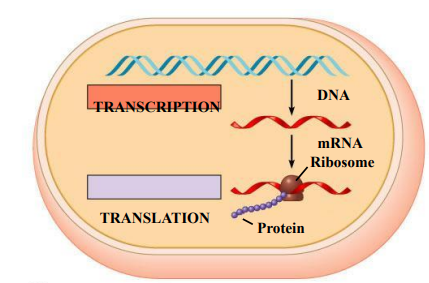
There is no nucleus, Everything happens in the cytoplasm
Eukaryotes
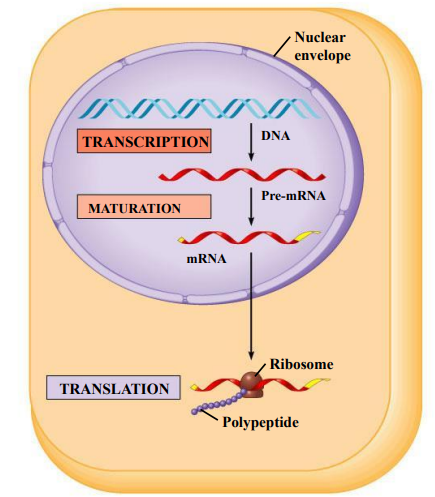
Transcription in the nucleus
RNA is exported from the nucleus to the cytoplasm
Translation of mRNA happens in the cytoplasm
Transcription: From DNA to RNA

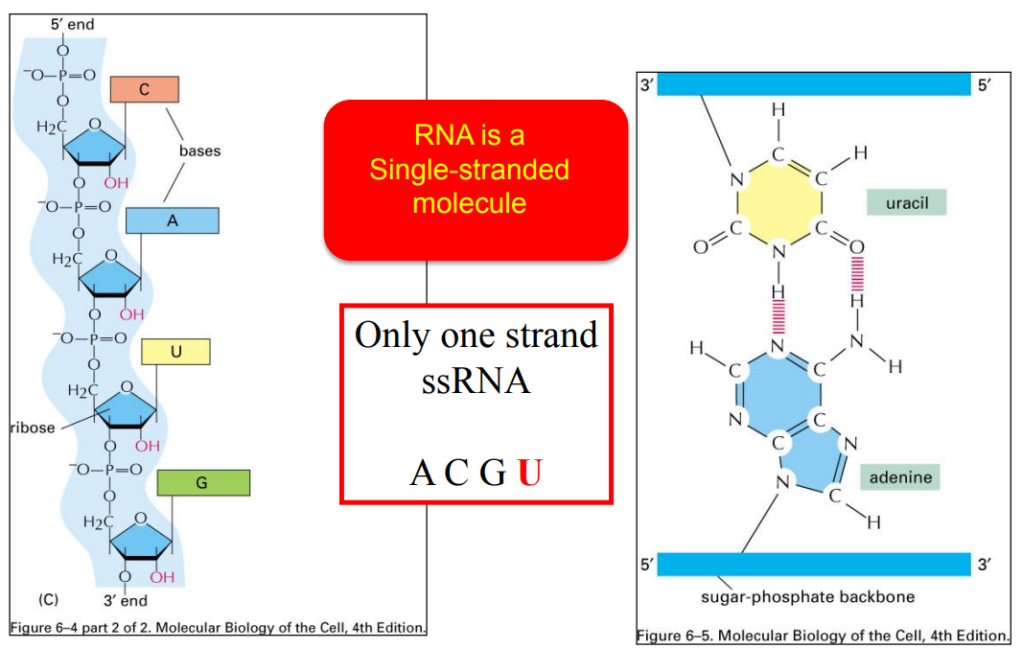
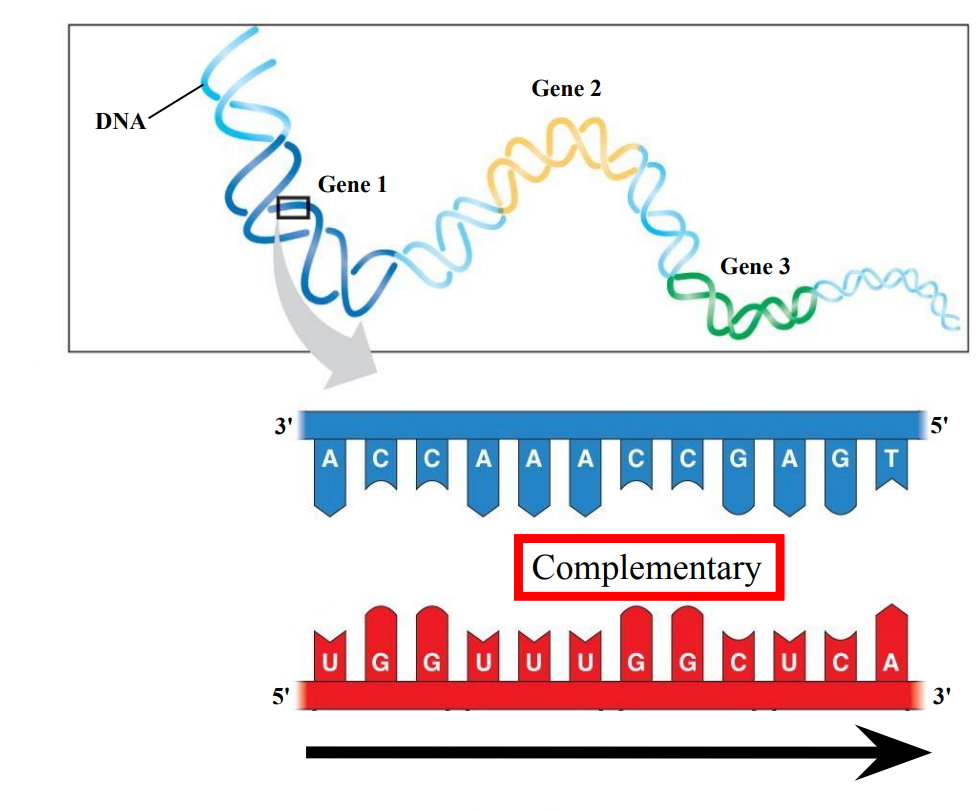
Complementarity
DNA RNA
A→ U
T →A
G →C
C→ G
DNA strand (template)
↓
TRANSCRIPTION
↓
RNA
Several RNA:
– ribosomal
– transfer
– small nuclear
– micro
– messenger
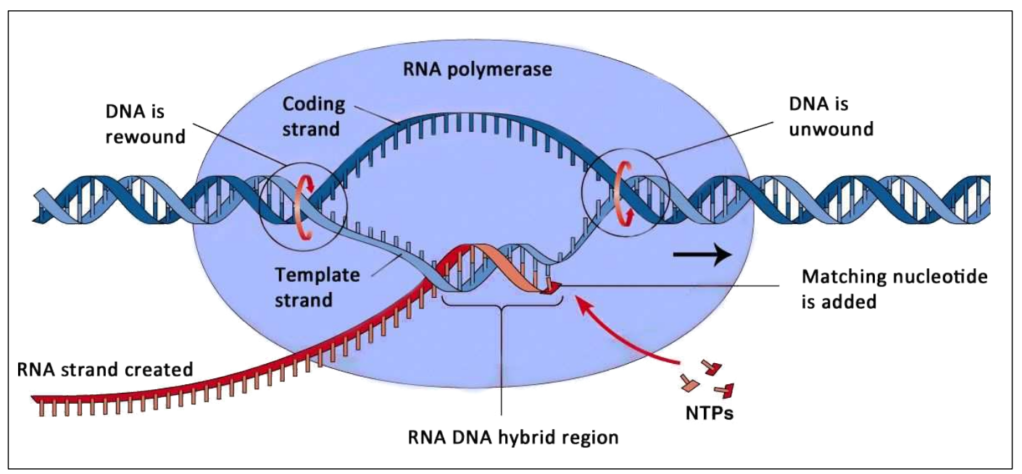
RNA Polymerase performs all steps



RNA strand Template strand Non-template strand |

Same dsDNA
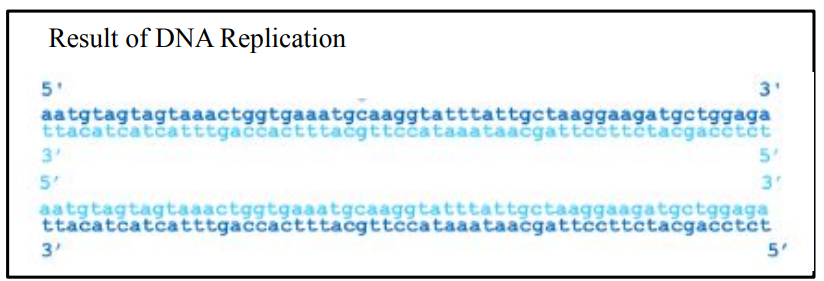
Original DNA
New strand

RNA
Template strand
Non-template strand
DNA contains the genetic information used to produce proteins

Transcription is the first step for the expression of genes
Translation: From mRNA to polypeptide
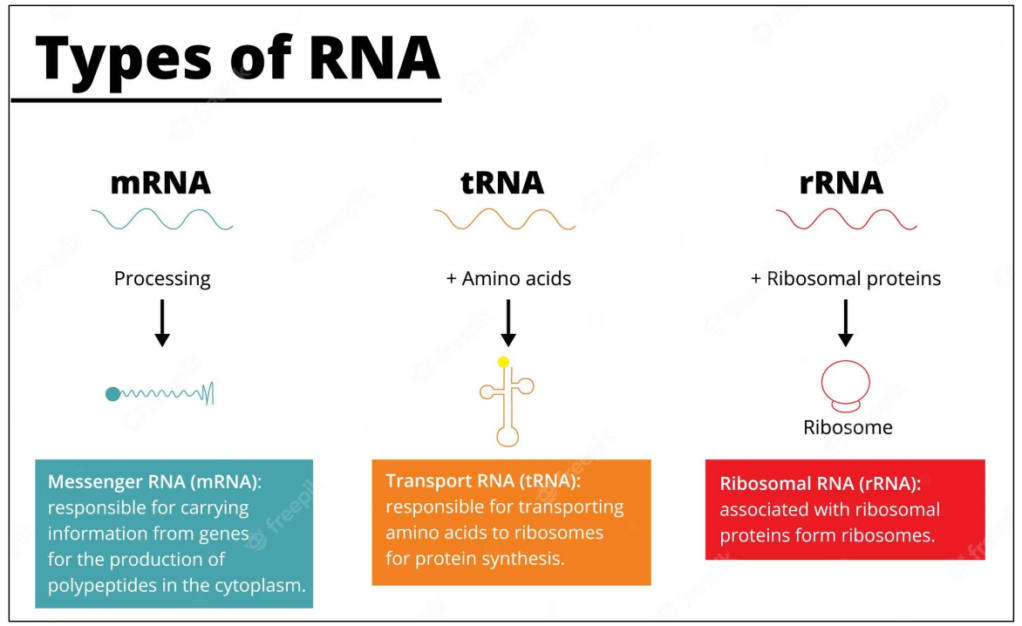
There are many types of RNAs
tRNA = transfer RNA
rRNA = Ribosomal RNA
mRNA = Messenger RNA
etc…
ONLY mRNA are used in translation
Goal of translation ?
Use the information in mRNA to make protein = Translate the sequence in mRNA to know the sequence of protein that has to be made
Why the word “Translation” ?
From one language to a different language
From “mRNA language” to “protein language”
ACUGAUCGGGCUAGCUAGC ………… M P V A T W G
mRNA alphabet is composed of FOUR letters, ACGU = Nucleotides
Protein alphabet is composed of 20 “letters”, ARNDCQEGHILKMFPSTWYW = Amino acids
Three ways to write amino acids
alanine – ala – A arginine – arg – R asparagine – asn – N
aspartic acid – asp – D cysteine – cys – C glutamine – gln – Q
glutamic acid – glu – E glycine – gly – G histidine – his – H
isoleucine – ile – I leucine – leu – L lysine – lys – K
methionine – met – M phenylalanine – phe – F proline – pro – P
serine – ser – S threonine – thr – T tryptophan – trp – W
tyrosine – tyr – Y valine – val – V
How to know which amino acid is needed
Out of 20 possibilities
When we can only use nucleotides
Out of only 4 nucleotides ???GE
We need a CODE
The Genetic code
Genetic code
Four different bases are used to be translated into a combination of 20 different amino acids
Need for 20 different combinations of bases . There are 4 different bases
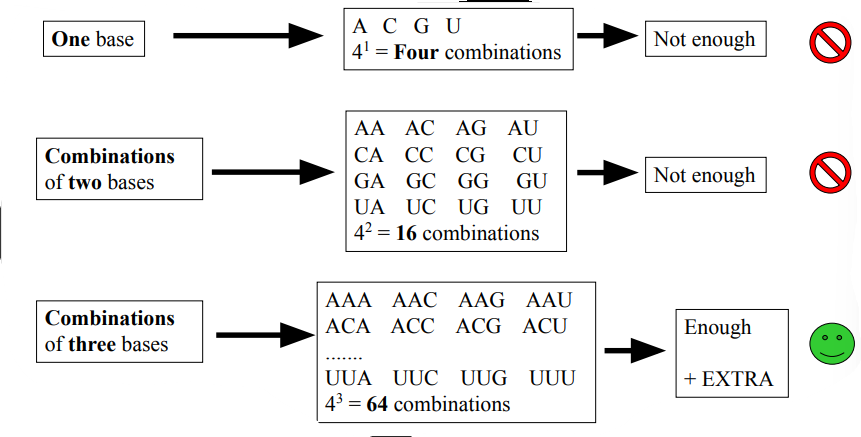
One combination of three bases = one mRNA codon



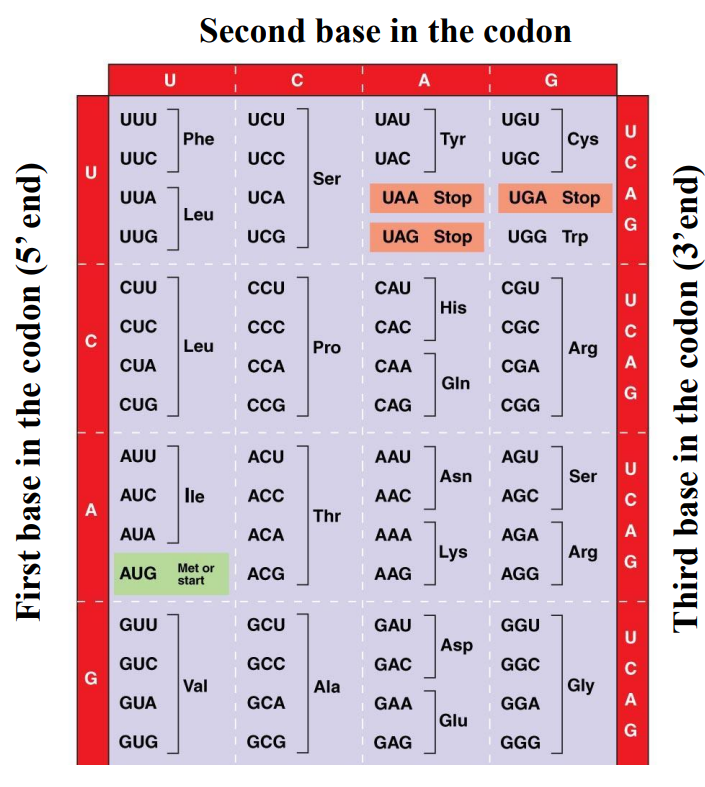
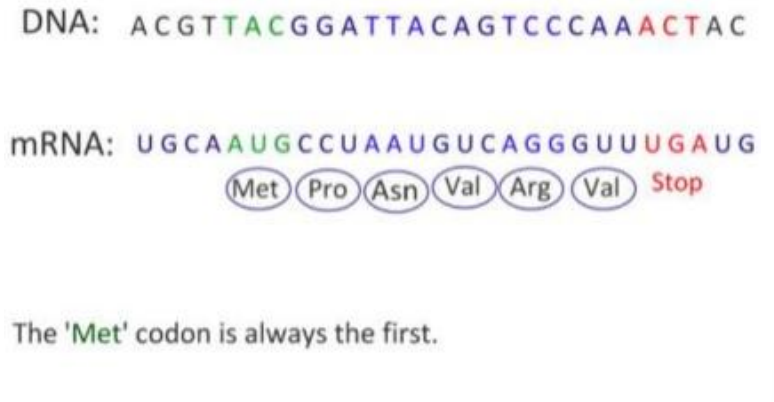
How to use the genetic code?
1. Read the mRNA sequence codon by codon
2. Find the corresponding amino acid
Codon UUU …… amino acid Phe (Phenylalanine)
Codon GAU …… amino acid Asp (Aspartic acid)
Codon AUG …… amino acid Met or Start
(methionine or ???)
Codon UAA …… Stop (???)
Codon UGA …… Stop (???)
Codon UAG …… Stop (???)
The mRNA is read “3 bases at the time”
Three mRNA bases = one codon/triplet
One codon always codes for the same amino acid
The genetic code is non-overlapping
The genetic code is degenerate
The genetic code contains punctuation codons
The genetic code is (almost) universal
- The genetic code is non-overlapping
In a mRNA molecule, codons are consecutive
They are read one by one, one after the other, in a continuous manner
A base is never part of two different codons
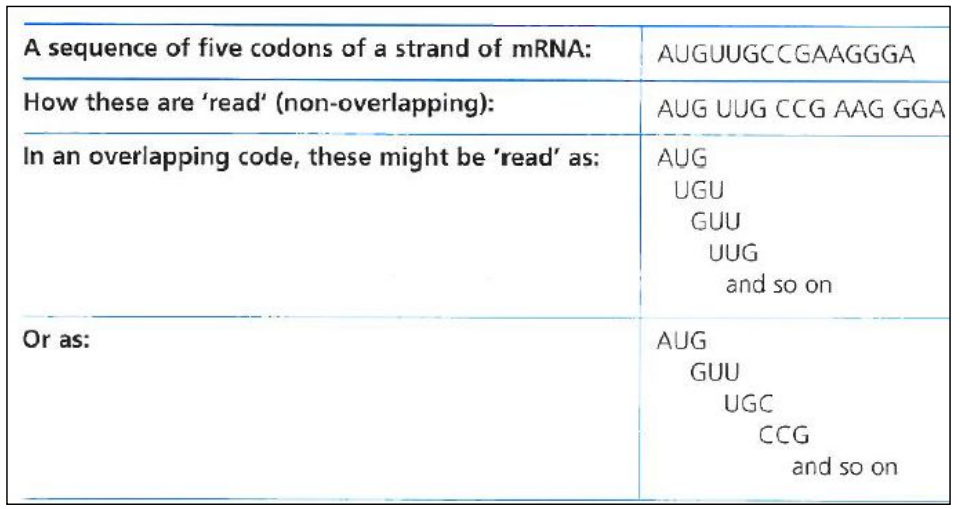
- The genetic code is degenerate
One amino acid may be encoded by more than one codon
The genetic code requires at least 20 codons minimum
It contains 64 codons
Contains more information than required
While Methionine and Tryptophane are encoded by single codons
All other amino acids are encoded by more than one codon
“Degeneracy” = the third base of the codon looks less important than
the first and second bases
e.g. Phenylalanine encoded by UUU and UUC
e.g. Proline encoded by CCU, CCC, CCA and CCG
- The genetic code contains punctuation codons
The Start/Methionine codon
The codon AUG encodes the amino acid Methionine
AND
The codon AUG marks the position where translation starts
CONFUSION ?
What if a mRNA contains several AUG codons ?
Start translation at each AUG?
Multiple proteins out of one single mRNA?
Only the first AUG is THE start codon
Any other AUG is used for Methionine
All proteins have Methionine as their first amino acid
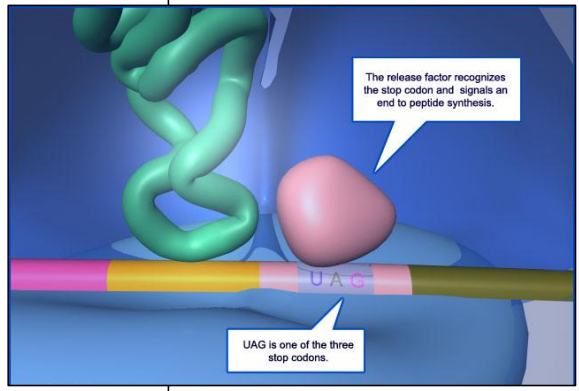
The 3 stop codons
Three codons do not encode any amino acids UAA UAG UGA
If they are “read” by the ribosome, translation will end
They do not attract any tRNA+amino acid
They attract a release factor = a protein that can fit in the ribosome where new tRNA+amino acid enter the ribosome
Presence of release factor in ribosome ends translation
The last amino acid is encoded by the codon just before the stop codon
How many amino acids in the polypeptide?
- The genetic code is (almost) universal
(almost)* all organisms use the same genetic code
A human mRNA is translated into the corresponding protein
In a cat’s cell, this human mRNA is translated into the same protein
In a carrot’s cell, this human mRNA is translated into the same protein
In a yeast’s cell, this human mRNA is translated into the same protein
The genetic code is the same in those species
The genetic code is universal
REALLY?
Codon UGA in human’s mRNA: Stop codon
Codon UGA in Mitochondria’s mRNA: Trp (Tryptophane)
Codon UGA in Paramecium’s mRNA: Gln (Glutamine)
The same codon can be read different ways
Different genetic codes
Not all species/organelles use the same genetic code
The genetic code is NOT universal
It is almost universal
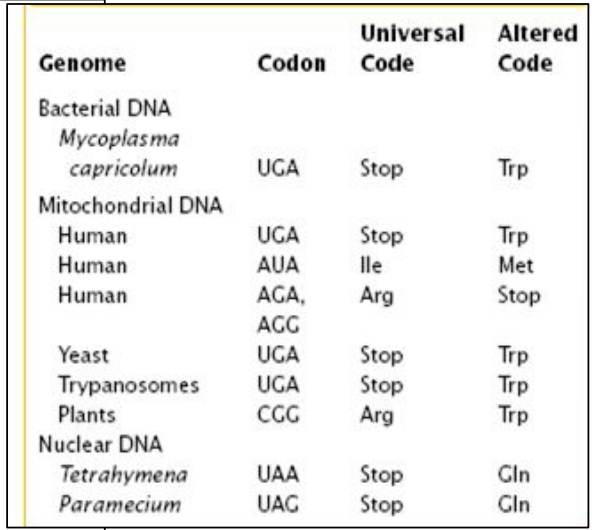
Many organisms use the same genetic code
Except some … exceptions
Consequence: Transgenic organisms are possible
Gene A from organism A encoding protein A
Transferred into organism B = genetic transformation
Organism B is now transgenic for gene A from organism A
Organism B will produce protein A from gene A
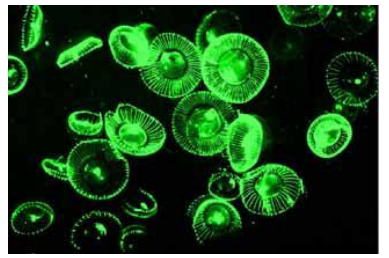
Jellyfish is green fluorescent
Due to GFP protein
Green Fluorescent Protein
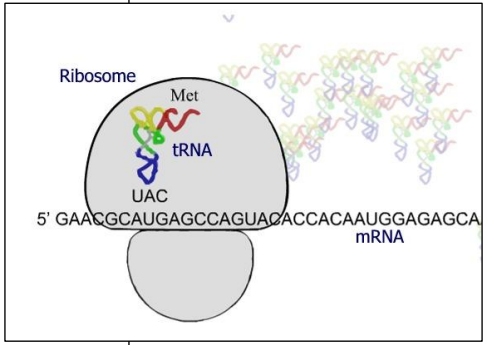
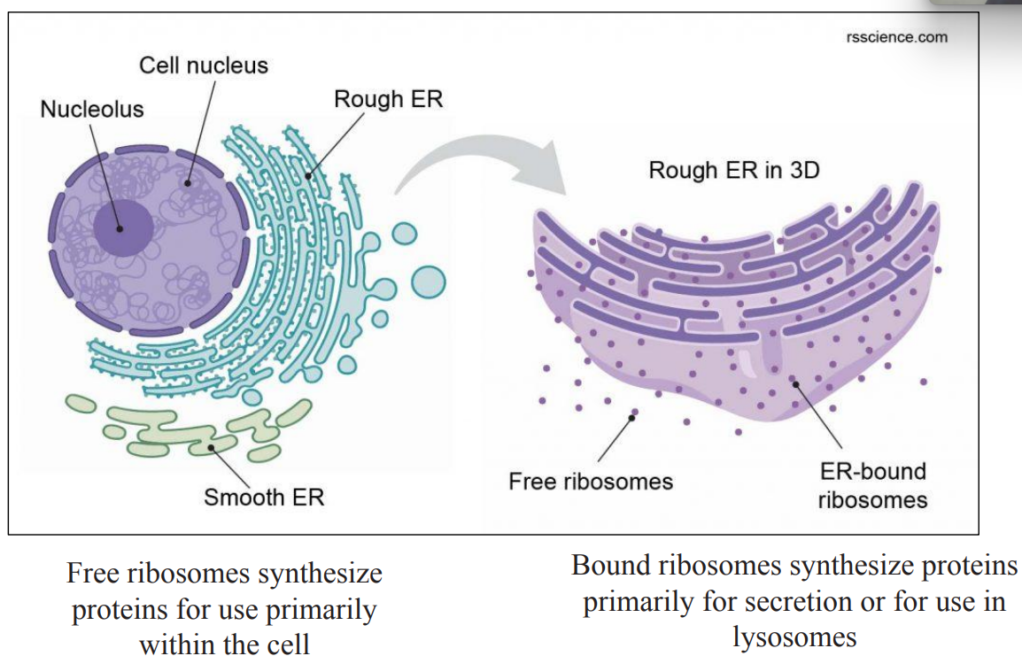
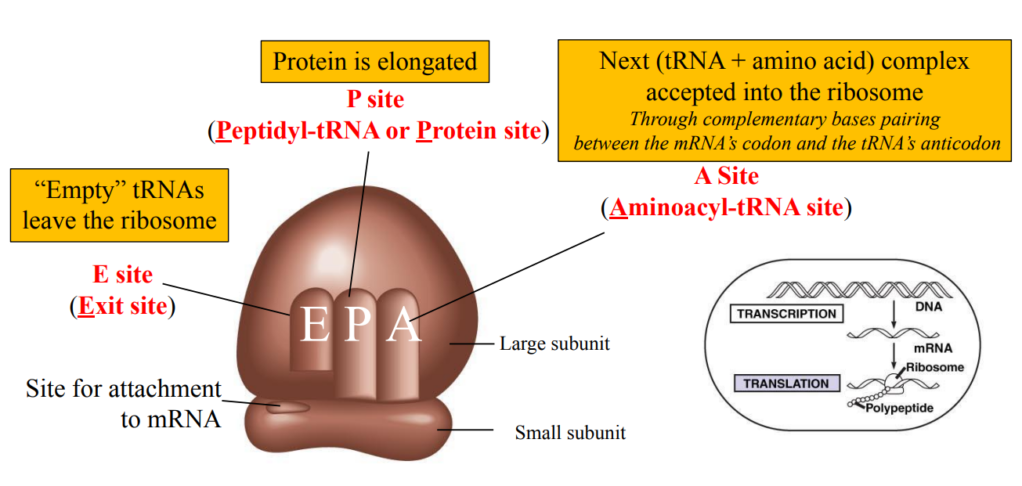
Ribosomes
Ribosome = Site of translation
Bacteria, mitochondria, chloroplasts
– “smaller”
20 nm diameter
Eukaryotes
– “bigger”
25-30 nm diameter

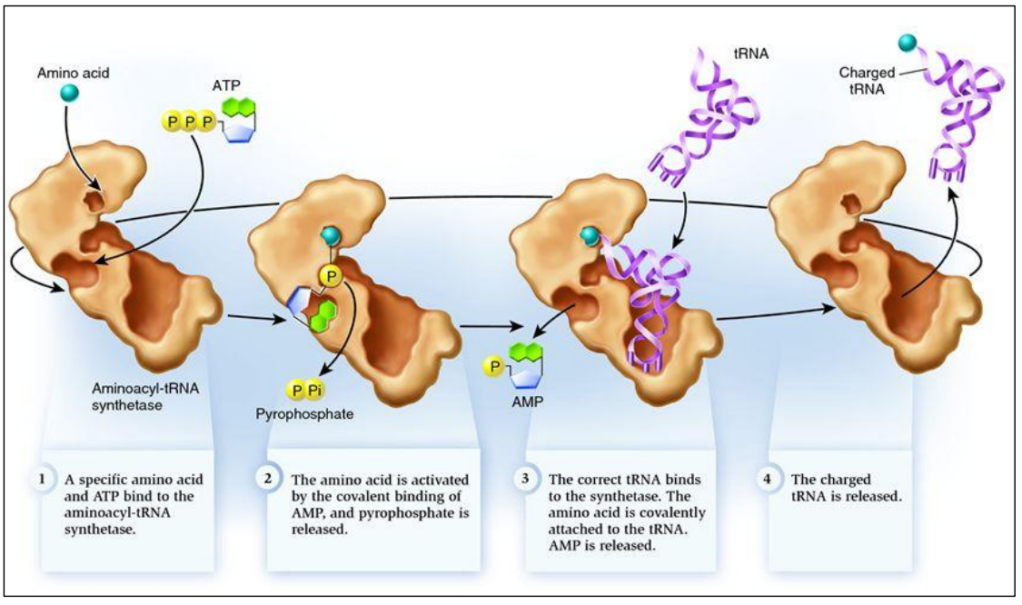
tRNA = intermediate between mRNA and amino acids
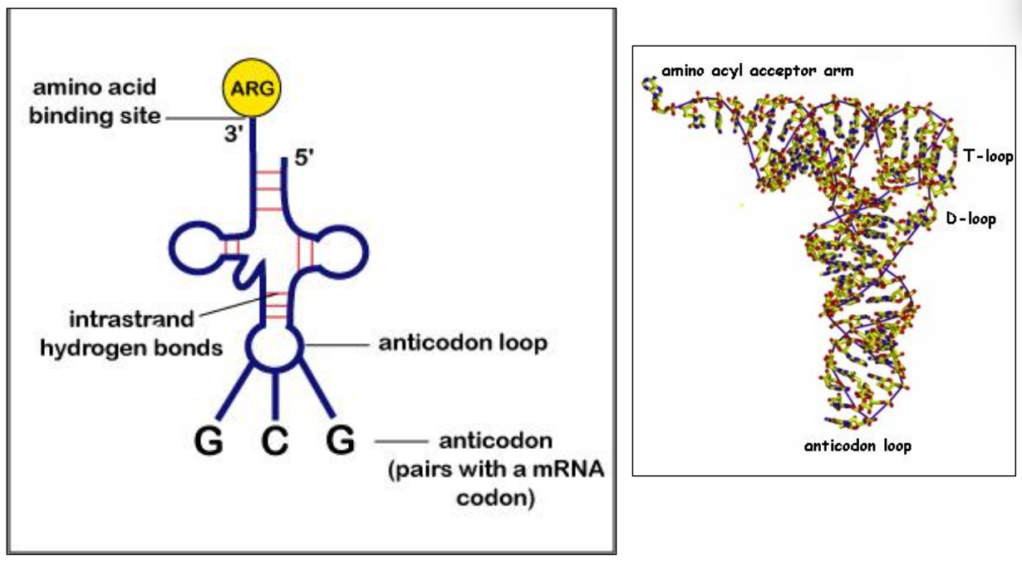
tRNA + amino acid = aminoacyl-tRNA
tRNA = intermediate between mRNA and amino acids
tRNA-activating enzymes ensure the right amino acid is linked to the right RNA
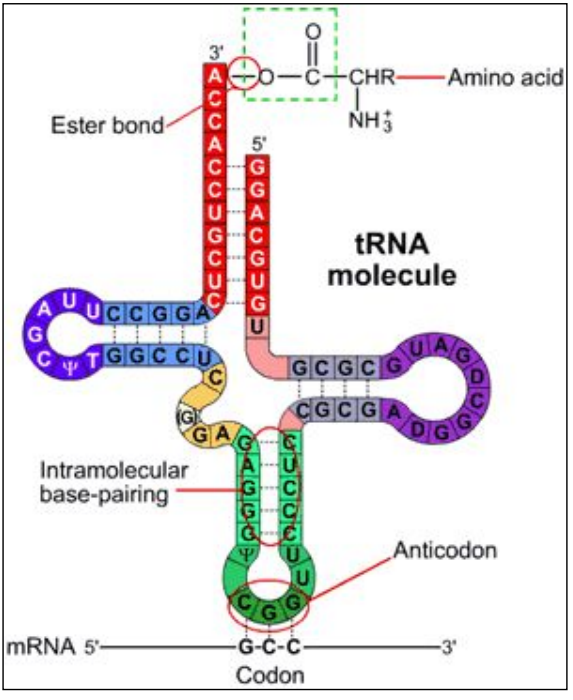
An anticodon is complementary to a codon
One amino acid can be encoded by
One codon (TRP) or More than one codon (LEU – 6 codons)
One amino acid will be attached to
One tRNA – one anticodon (TRP) Or More than one tRNA – more than one anticodon (LEU – 6 tRNAs – 6 anticodons)
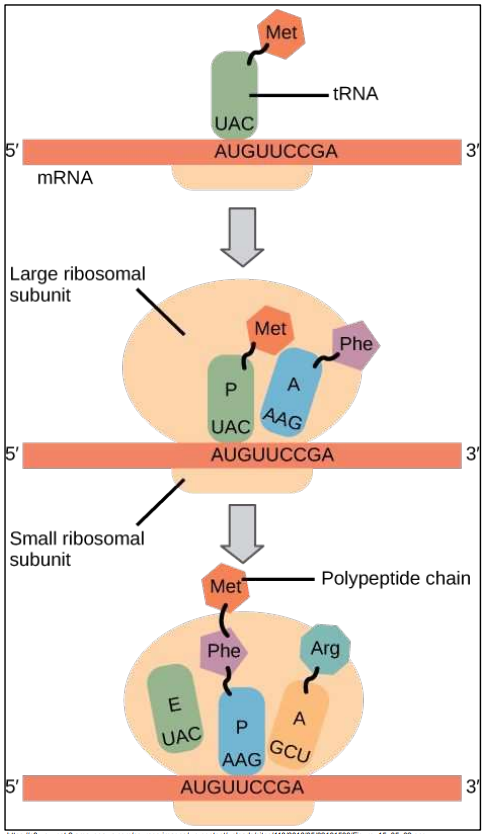
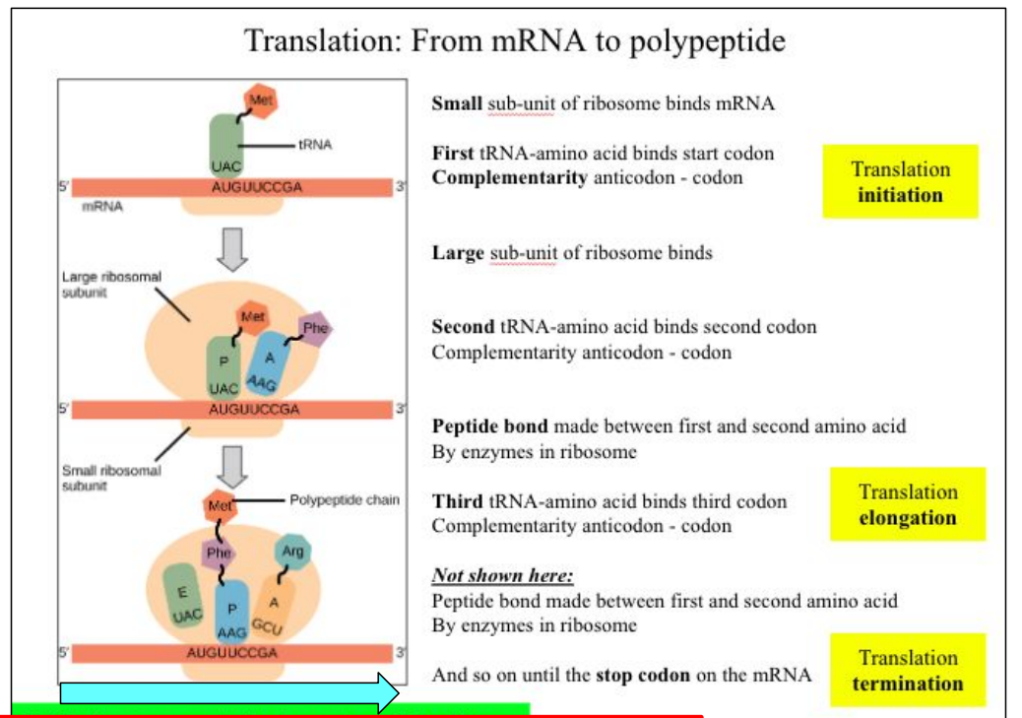
Small sub-unit of ribosome binds mRNA
First tRNA-amino acid binds start codon
Complementarity anticodon – codon
Large sub-unit of ribosome binds
Second tRNA-amino acid binds second codon
Complementarity anticodon – codon
Peptide bond made between first and second amino acid
By enzymes in ribosome
Third tRNA-amino acid binds third codon
Complementarity anticodon – codon
Not shown here:
Peptide bond made between first and second amino acid
By enzymes in ribosome
And so on until the stop codon on the mRNA
Mutations that change protein structure
What is a DNA mutation?
A mutation is a permanent* change in a DNA sequence
What types of DNA mutations are there?
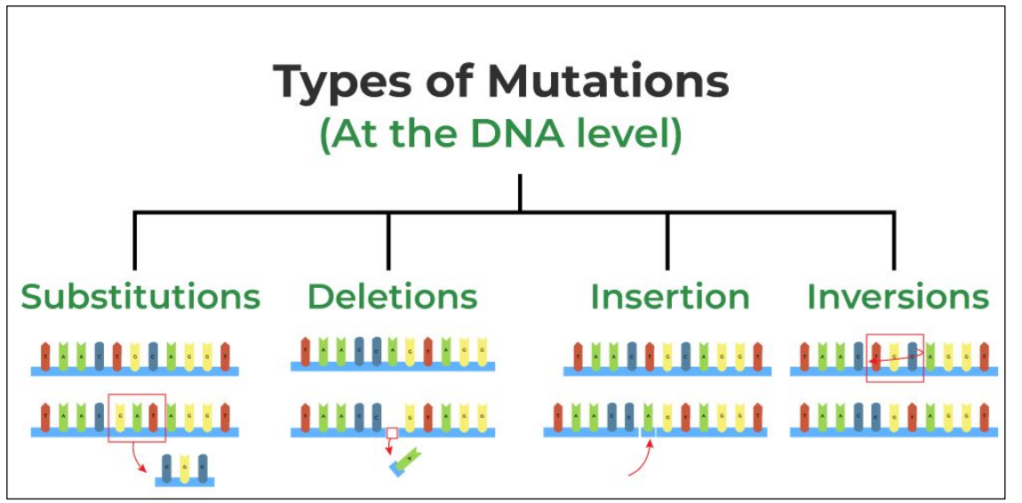
A point mutation = a single base is changed substituted, deleted, inserted
What consequences do DNA mutations have on protein sequence and structure?
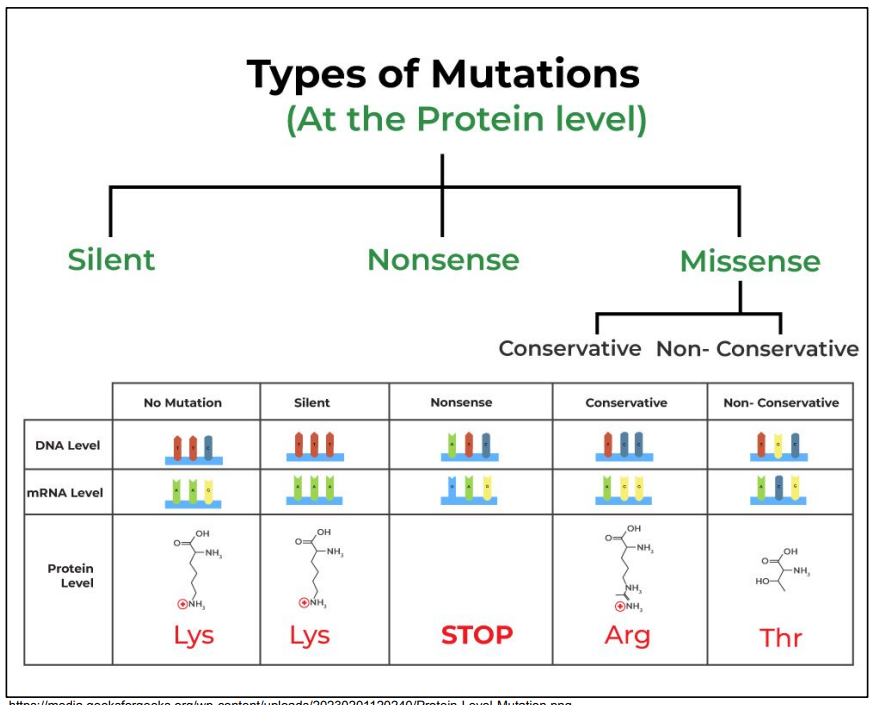
Silent
Genetic code is degenerate
Several codons can code for the same amino acid
Protein sequence unchanged
Protein structure unchanged
Nonsense
Genetic code contains punctuation codons
Protein sequence shorter
Protein structure may be changed
Conservative
Protein sequence changed
Protein structure may be changed
Non-Conservative
Protein sequence changed
Protein structure probably changed
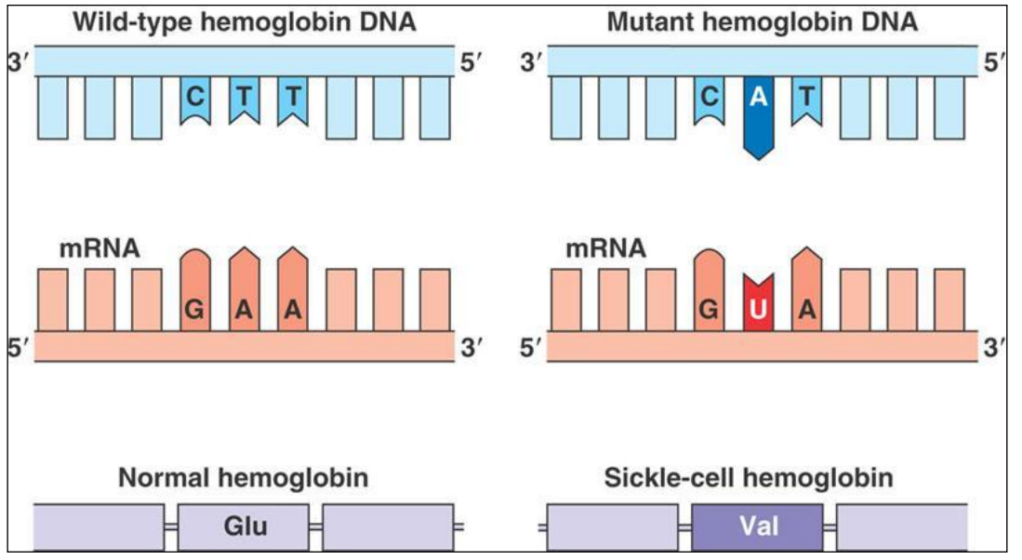
Sickle-cell anemia
What is anemia?
Anemia = Decrease in Red Blood Cells’ number OR in Haemoglobin content
– less oxygen is transported from lungs to body cells


Sickle-cell Anemia = anemia due to sickled red blood cells
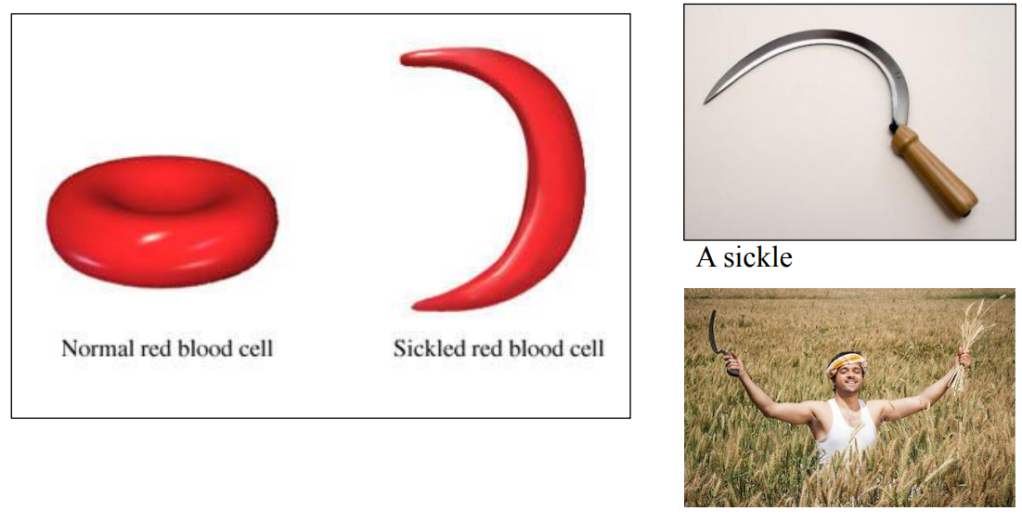
Haemoglobin and Sickle-cell Anemia
A SINGLE amino acid in Haemoglobin is “wrong”


Non-coding sequences in DNA (HL only)
Regulators of transcription: Promoters and Terminators
Structure of eukaryotic genes
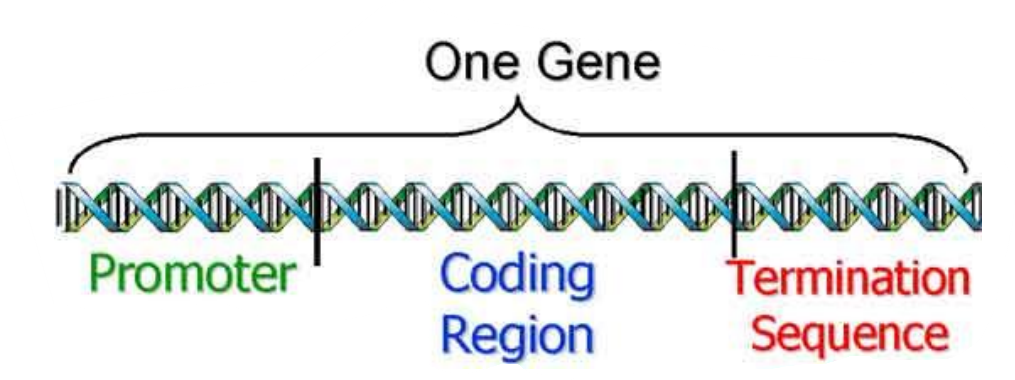
Promoter
“Wait my signal before you start reading!”
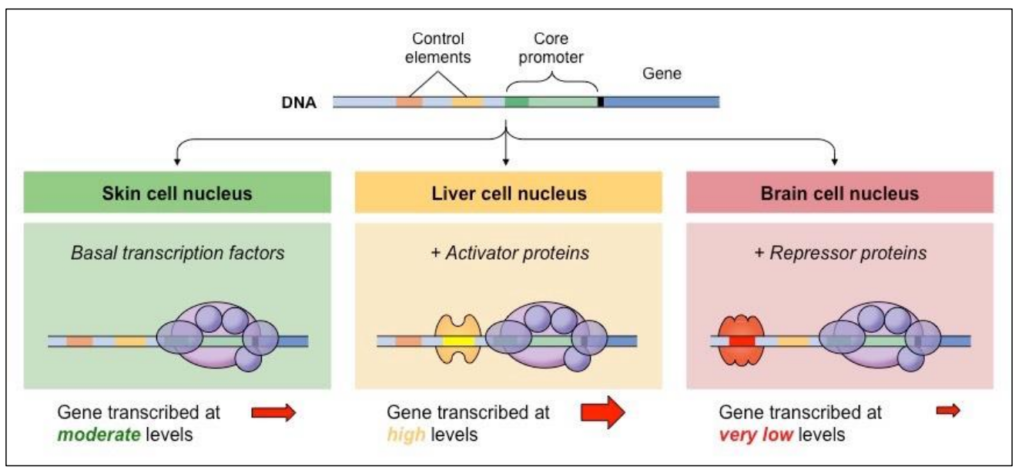
Inactive/Active
Activated only if specific transcription factors bind these base pairs
Coding region
“read the information!”
Contains the bases that encode the amino acids of the protein
Termination sequence
“Stop reading!”
Contains the base pairs that make transcription cease
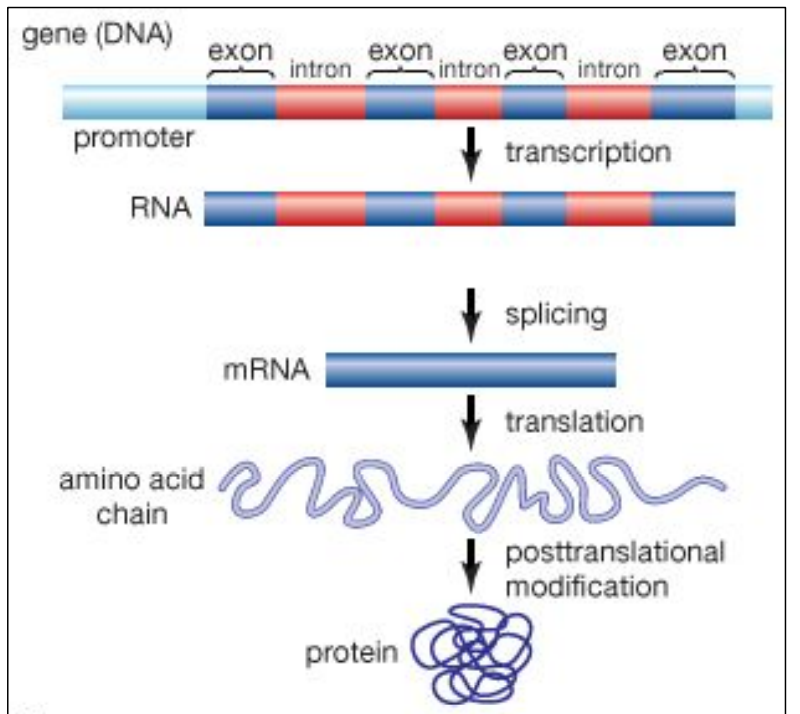
Introns and exons in eukaryotic genes
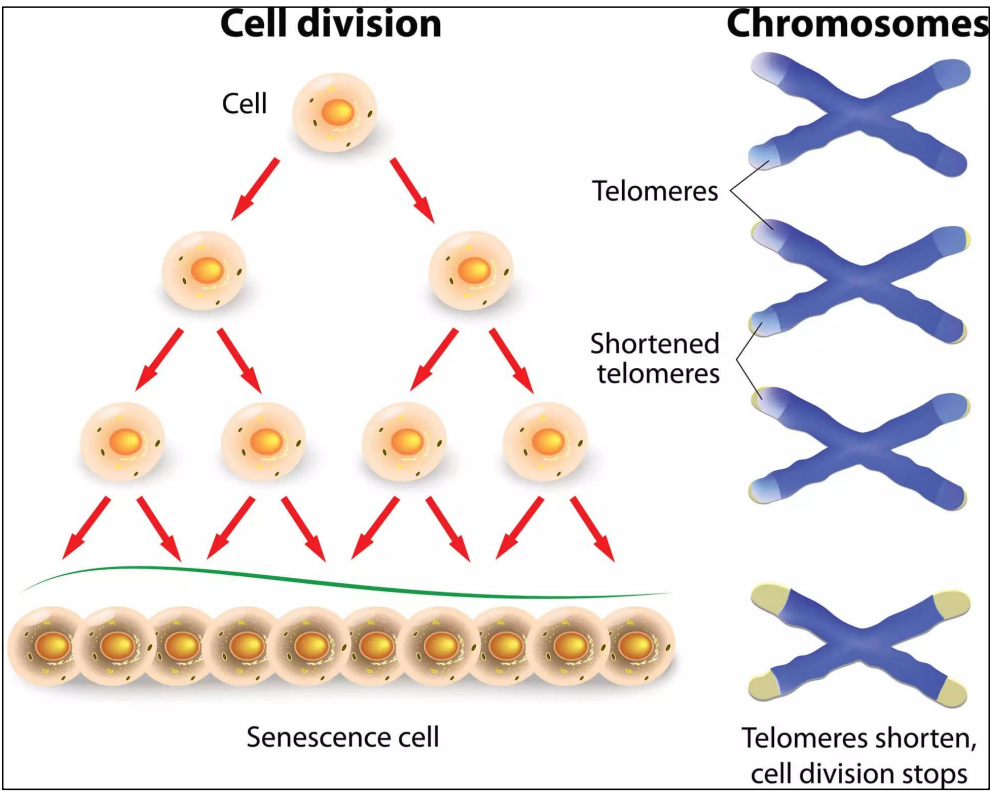
Telomeres shortening limits cell division
Only mRNAs code for proteins
Directionality of transcription and translation (HL only)




From 5’ end to 3’ end of the strand = RNA strand
Initiation of transcription (HL only)
Transcription factors (Activators, Repressor) bind promoters

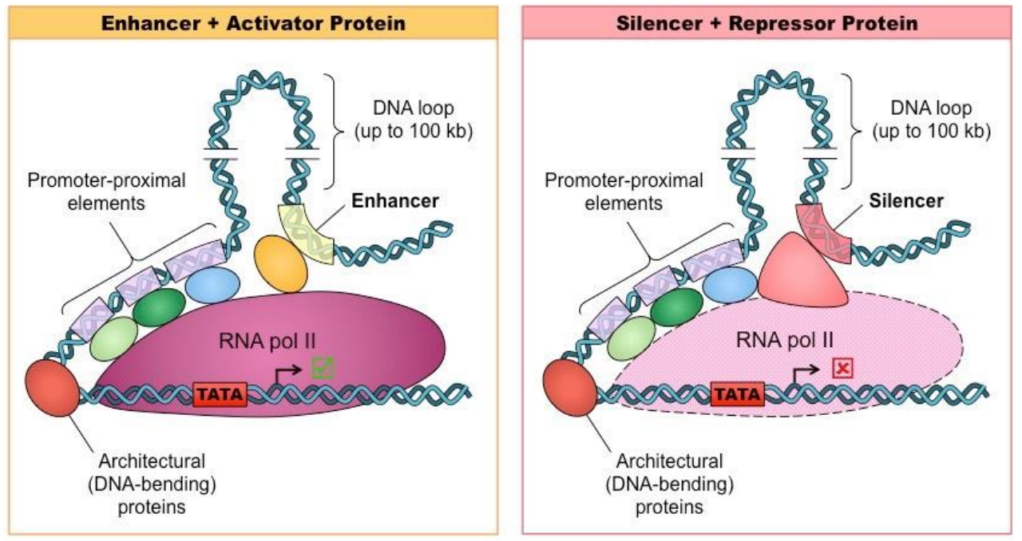
Transcription factors bind promoters in eukaryotic genes
Post-transcriptional modifications in eukaryotic cells (HL only)
In eukaryotes, mRNA undergoes modifications after transcription, before translation
“Post-transcriptional modifications”
IN THE NUCLEUS
Immediately after transcription, mRNA is called pre-mRNA / immature mRNA
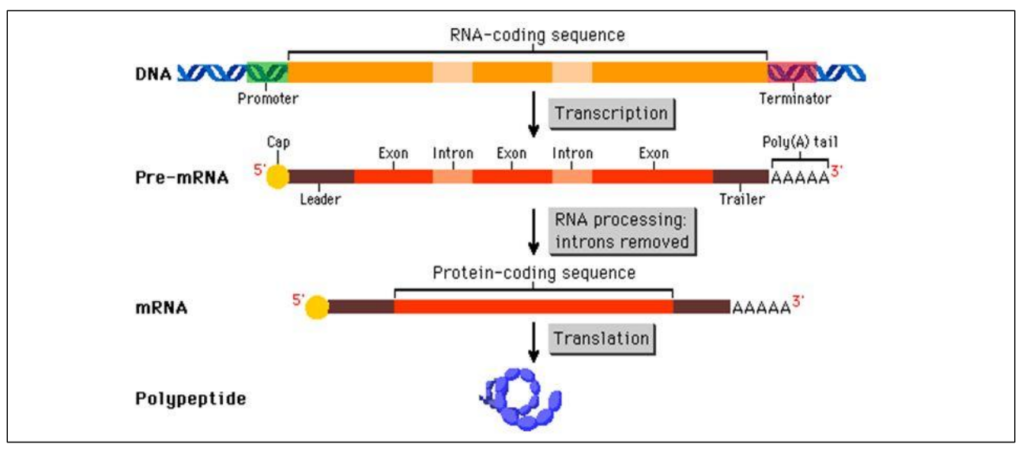
Post-transcriptional modifications of pre-mRNA
1. Addition of 5’ Cap Protection from RNAses
2. Addition of 3’ PolyA tail Protection from RNAses + enhances translation
3. Splicing of exons Multiplies number of possible polypeptides
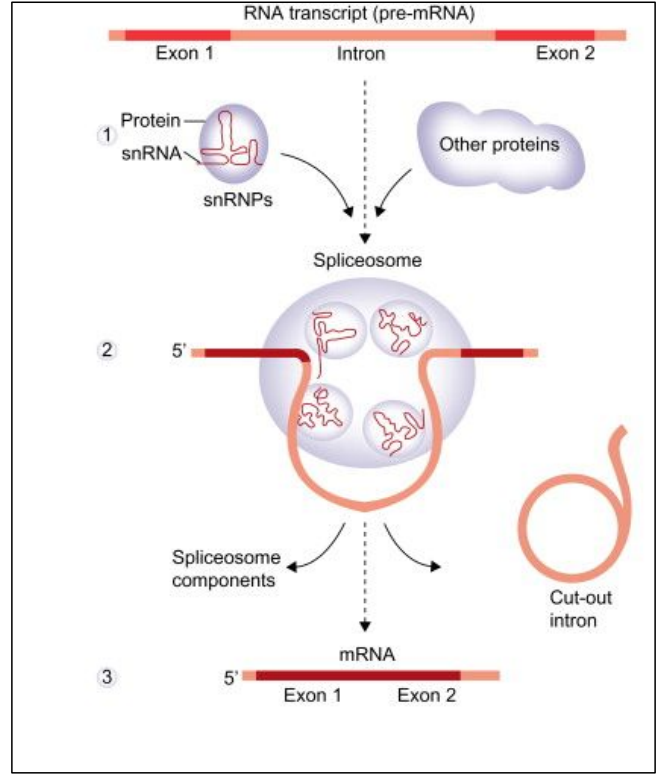
“Splicing” = joining two linear pieces together
Most eukaryotic genes are discontinuous
The coding region of the gene is composed of two elements
– Exons = used in final/mature mRNA
– Introns = NOT present in final/mature mRNA
But both exons and introns are present in the initial/immature mRNA
Introns will be “eliminated” / spliced out
Exons will be spliced together
Mature mRNA is composed of exons only
Only exons contains the codons
Only exons are coding for proteins

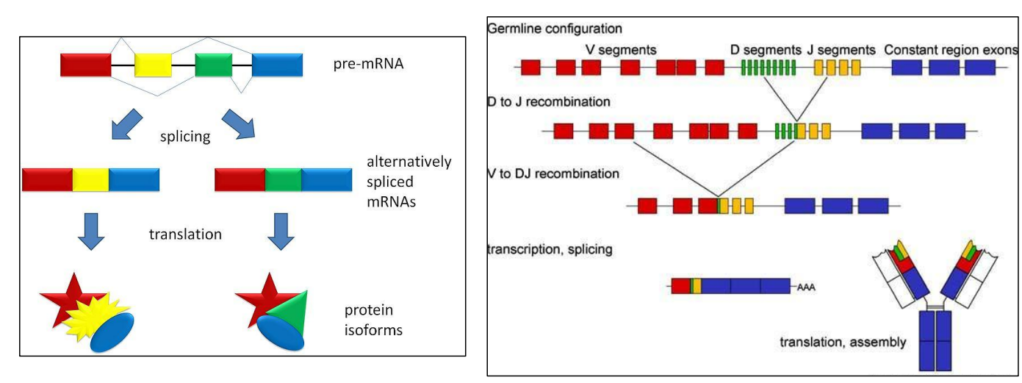
Alternative splicing
Human body can make antibodies that are
– embedded in the membrane of Lymphocyte B
– secreted by lymphocytes B
Two forms of the same proteins making up antibodies
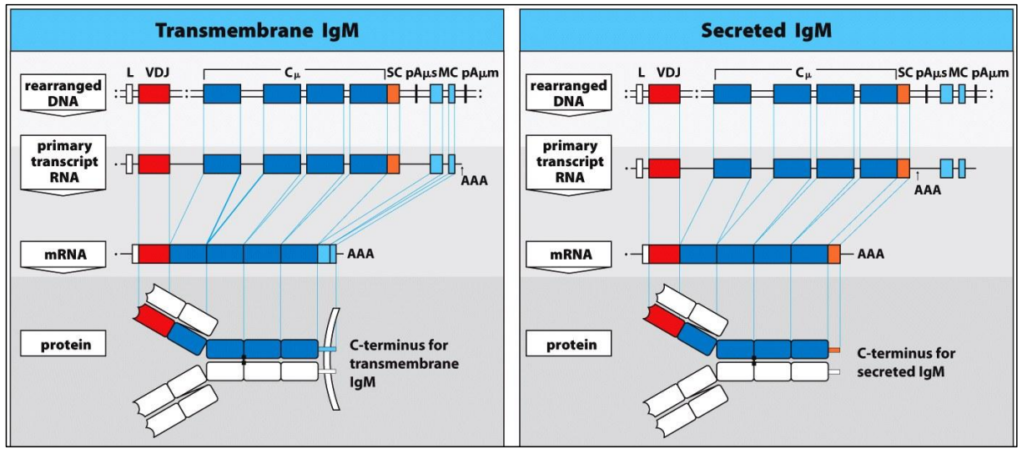
Human genome contains less than 30 000 genes
Human body can produce …. 10 billions of different antibodies!!!
Several mechanisms to increase the number of antibodies
One is alternative splicing
Three steps of translation (HL only)
Translation comprises three steps
1. Translation initiation
Starting the translation of an mRNA
Bringing the first amino acid = Methionine
2. Translation elongation
Keep bringing and linking together amino acids
= making the protein longer
As long as there are codons in the mRNA
3. Translation termination
The last codon has been read
The stop codon is read
Stop translation
Release all actors of translation
Translation initiation

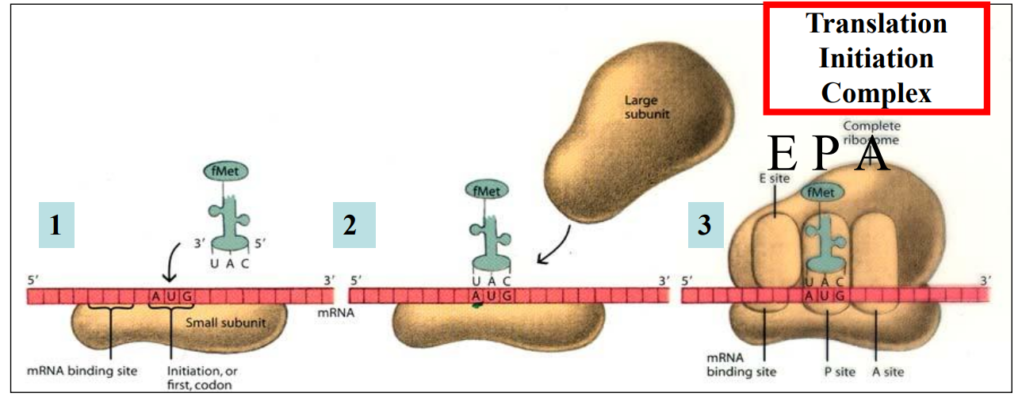
1. Ribosome’s small subunit attaches to mRNA by complementarity between rRNA and mRNA
Start codon “attracts” the first aminoacyl-tRNA through complementary base pairing codon anticodon
2. The first aminoacyl-tRNA sticks tothe start codon via its anticodon
Ribosome’s large subunit attaches to the small subunit
3. The first aminoacyl-tRNA and the start codon are in the P site
Translation elongation
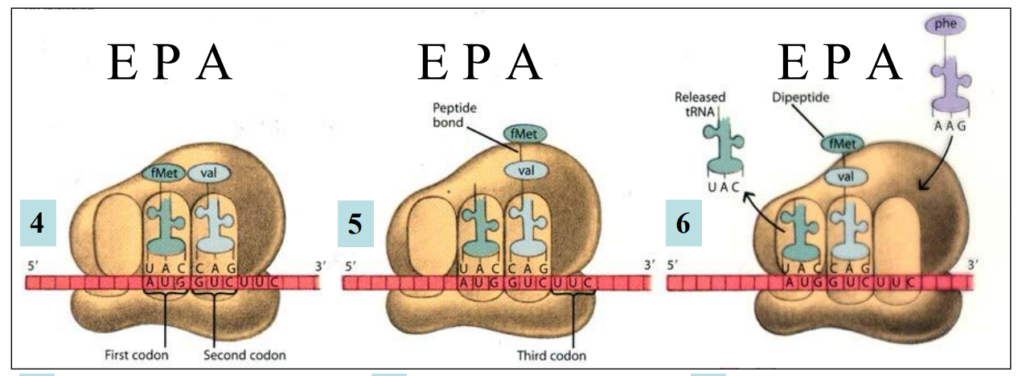
4. The second codon “attracts” the anticodon of the second aminoacyl-tRNA through complementary base pairing codon-anticodon In the A site
5. The two first amino acids get linked together by a Peptide bond through a condensation reaction
Dipeptide is formed in P site
Bond between the first tRNA and the first amino acid is broken through an hydrolysis reaction
6. Translocation of the Ribosome
First tRNA in E site: exits
Second tRNA + dipeptide in P site
A site ready to accept the third aminoacyl-tRNA
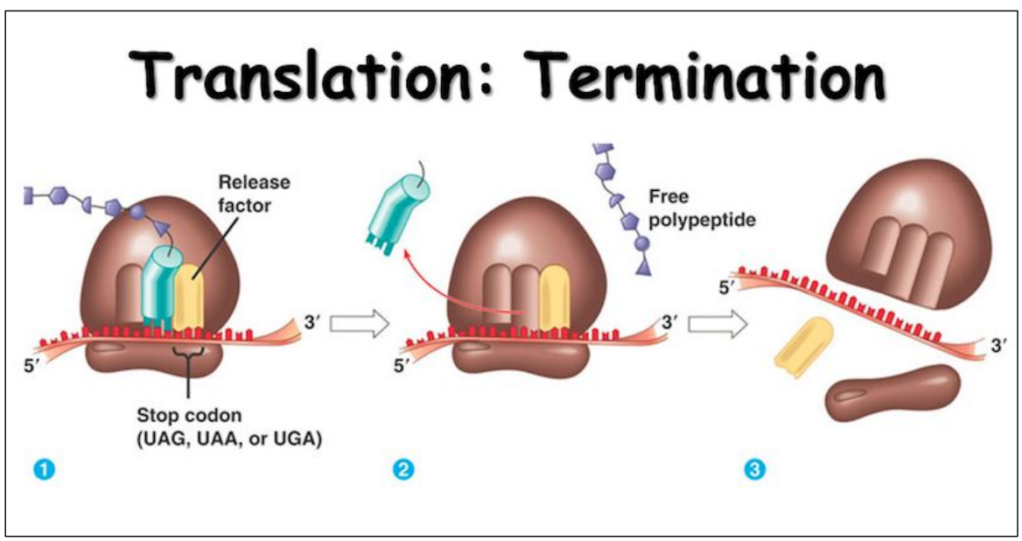
Translation termination
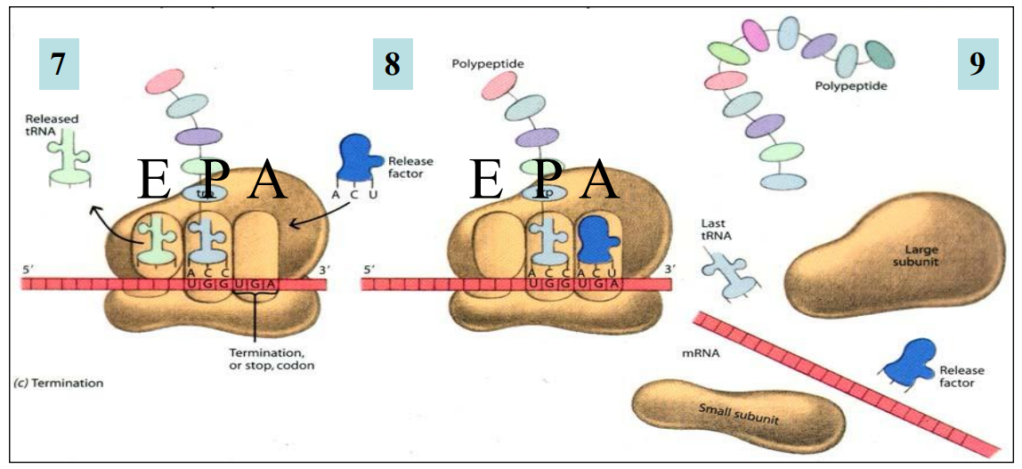
7. Stop codon “attracts”
RELEASE FACTOR (a protein) through recognition of the stop codon by specific amino acids in the release factor
No amino acid
8. Release factor in A site
9. Translation complex disassembled
All components released
mRNA can be translated again
The three release factors look like tRNAs
Post-translational modifications (HL only)

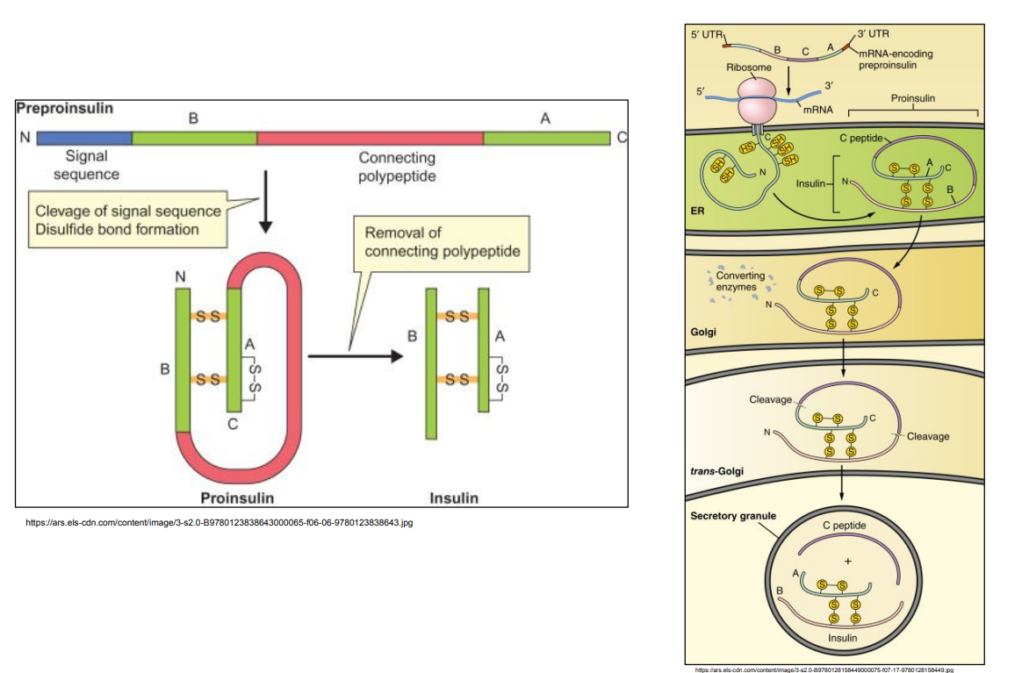
Hormone insulin released into blood when glucose concentration is high
– Opens glucose transporter
– Glucose enters cells for respiration
– Blood glucose concentration decreases
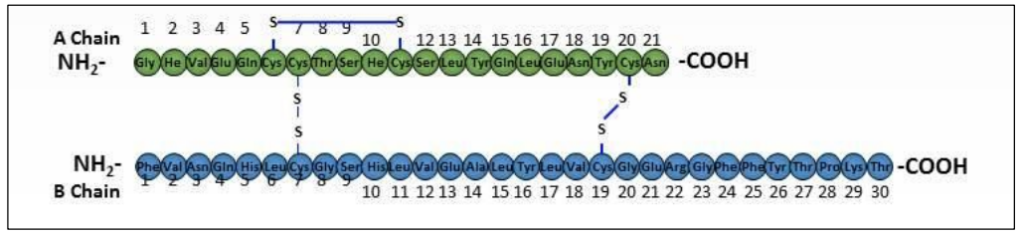
Insulin is coded by a single gene
Insulin protein = Two polypeptide chains (quaternary structure)
Recycling of amino acids by proteasomes (HL only)
Proteome = set of all proteins
Proteome of a cell = only proteins needed by this cell
– Proteome of a tissue is the same as an individual cell of this tissue
Tissue = group of cells specialized the same way
– Proteome of a cell is smaller than
an organ’s proteome
Organ = group of different tissues
– Proteome of a cell is smaller than
an organism’s proteome
Organism = sum of all organ systems

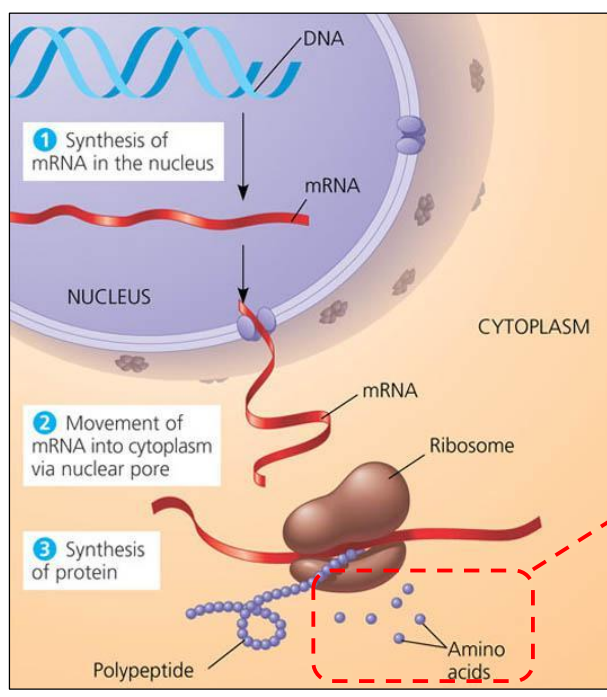
Amino acids = units of polypeptides
– Need a pool of them for translation
Polypeptide
Proteins not needed anymore ,are a waste of energy
– Digested by proteases
Amino acids recycled
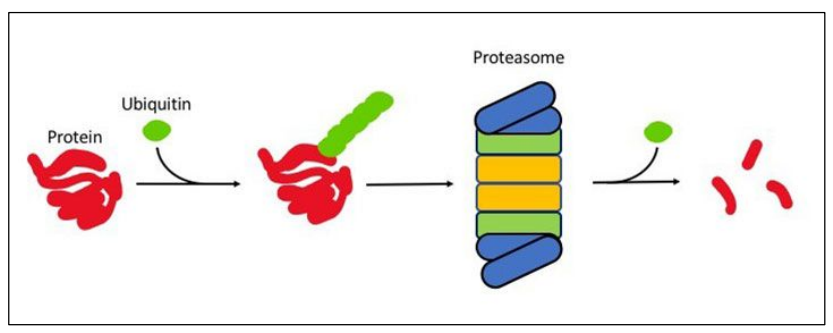
Main process to recycle amino acids = Proteasome
Proteome = protein synthesis – protein breakdown
Ubiquitin recruits non-needed proteins
Brings them to the proteasome = complex of proteases
– Proteins digested into amino acids
– Amino acids available for protein synthesis