14.1 Further aspects of covalent bonding and structure
Essential Idea:
Larger structures and more in-depth explanations of bonding systems often require more sophisticated concepts and theories of bonding.
Understandings:
- Covalent bonds result from the overlap of atomic orbitals. A sigma bond (σ) is formed by the direct head-on/end-to-end overlap of atomic orbitals, resulting in electron density concentrated between the nuclei of the bonding atoms. A pi bond (π) is formed by the sideways overlap of atomic orbitals, resulting in electron density above and below the plane of the nuclei of the bonding atoms.
- Formal charge (FC) can be used to decide which Lewis (electron dot) structure is preferred from several. The FC is the charge an atom would have if all atoms in the molecule had the same electronegativity.
- FC = (Number of valence electrons)-½(Number of bonding electrons)-(Number of non-bonding electrons).
- The Lewis (electron dot) structure with the atoms having FC values closest to zero is preferred.
- Exceptions to the octet rule include some species having incomplete octets and expanded octets.
- Delocalization involves electrons that are shared by/between all atoms in a molecule or ion as opposed to being localized between a pair of atoms.
- Resonance involves using two or more Lewis (electron dot) structures to represent a particular molecule or ion. A resonance structure is one of two or more alternative Lewis (electron dot) structures for a molecule or ion that cannot be described fully with one Lewis (electron dot) structure alone.
Applications and Skills:
- Prediction whether sigma (σ) or pi (π) bonds are formed from the linear combination of atomic orbitals.
- Deduction of the Lewis (electron dot) structures of molecules and ions showing all valence electrons for up to six electron pairs on each atom.
- Application of FC to ascertain which Lewis (electron dot) structure is preferred from different Lewis (electron dot) structures.
- Deduction using VSEPR theory of the electron domain geometry and molecular geometry with five and six electron domains and associated bond angles.
- Explanation of the wavelength of light required to dissociate oxygen and ozone.
- Description of the mechanism of the catalysis of ozone depletion when catalysed by CFCs and NOx.
14.1 Further aspects of covalent bonding and structure – Facts
• Sigma (σ) bonds form when atomic orbitals (s, p, or hybridized) overlap along the bond axis; all single bonds are σ bonds.
• Pi (π) bonds form when p atomic orbitals overlap laterally; the electron density is concentrated above and below the bond axis.
• Double bond = one σ bond and one π bond.
• Triple bond = one σ bond and two π bonds.
• Atoms in Period 3 and below can expand their octet using unoccupied d orbitals. This gives rise to molecules with 5 or 6 electron domains around the central atom.
• The number of resonance structures that can be drawn for a molecule is the same as the number of possible positions for a double bond.
• Delocalization of π electrons leads to greater stability and bonds of intermediate length and strength.
• Formal charge (FC) can be used to determine which of some possible structures is the preferred structure. The most stable structure is the one with the lowest values for formal charge for the atoms.
• FC = (number of valence electrons) –
[½(number of bonding electrons) – (number of non-bonding electrons)]
• Ozone is a resonance hybrid with a bond order of 1.5. Oxygen is a diatomic molecule with a bond order of 2. Ozone is therefore dissociated by light of longer wavelength.
• The catalytic breakdown of ozone by CFCs and NOx has contributed to significant depletion of the ozone layer.
The shared pair of electrons in a covalent bond is often depicted by a stroke, as in: H – Cl
This is an over-simplified version of what happens. As you know, 5 of the 7 electrons in the outer shell of the chlorine atom are distributed between 3 different p-orbitals. Two of the p-orbitals are filled with electrons and are not available for bond-forming. The third p-orbital is half-filled, and has a space that can be occupied by another electron. The hydrogen atom also has a half-filled orbital.
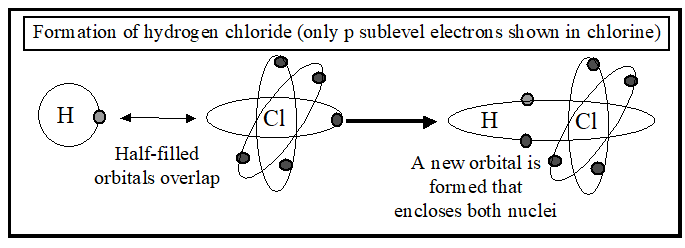
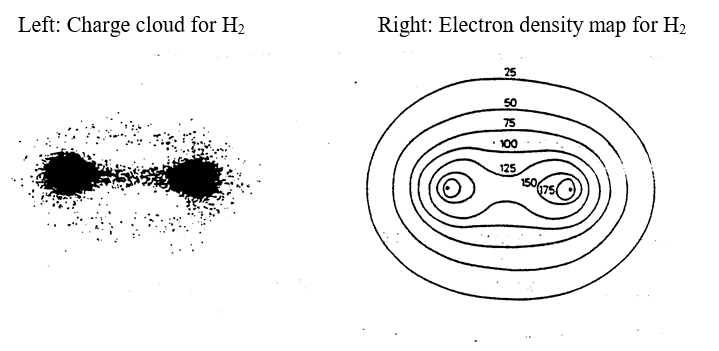
When hydrogen and chlorine react together, these two half-filled atomic orbitals overlap and join together to form a new kind of orbital (called a molecular orbital) that encloses both nuclei. The molecular orbital contains both the original electrons, which are now attracted to both nuclei. The attractive force between the 2 nuclei and 2 electrons in a molecular orbital is the covalent bond that holds the atoms together. The attractive force only acts between the nuclei and electrons inside the molecular orbital; it does not attract particles outside the molecular orbital. Unlike the ionic bond (where the attractive force radiates outward in all directions) a covalent bond is directional in nature.
It is possible to produce “pictures” of the molecular orbital in a hydrogen molecule from experiments that “map” the position of electrons at different times. The results look like these:
Dative Bonds: A Different Way of Forming a Covalent Bond
A covalent bond is formed when a pair of electrons is shared between two atoms. So far we have only considered the possibility that each atom contributes one electron to the shared pair, but there is another possibility. Sometimes one atom donates both electrons to the shared pair. Although the bond produced is identical to an ordinary covalent bond, we call it a dative bond or coordinate bond because it was formed in a different way.
In order to make a dative bond, one atom must have an orbital that contains two electrons that are not already involved in making bonds. This pair of electrons is referred to as a lone pair of electrons. Lone pairs of electrons are common in species involving non-metals and transition metals. The other atom must have an orbital that contains no electrons; this is referred to as an empty orbital. The most common case of a particle with an empty orbital is the hydrogen ion H+, which has no electrons in it’s s-orbital.
Look at the reaction between ammonia and hydrogen ions. In ammonia the nitrogen atom has a lone pair of electrons, while a hydrogen ion has an empty orbital. The hydrogen ion will be attracted to the negative charge on the lone pair of electrons. If the two particles collide, the lone pair of electrons (on the nitrogen atom) can move into the empty orbital on the hydrogen ion, and the two atoms end up sharing the electrons, resulting in a dative bond (ie, a covalent bond):
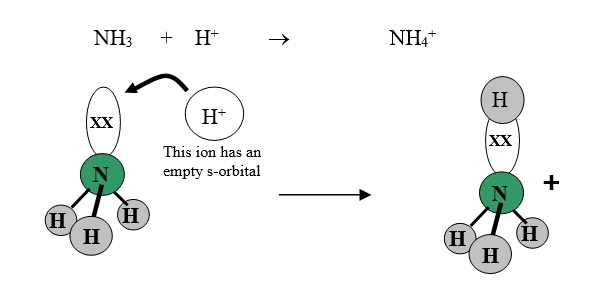
The ammonia molecule has been turned into an ammonium ion. (Note: students frequently confuse these two names and two formulae. It is worth memorising them !!!).
In diagrams, dative bonds are represented by an arrow, which shows the direction in which the donation of electrons occurs; the arrow-tip points towards the atom that originally had the empty orbital. A second (extremely common) example of dative-bond formation is the reaction that occurs whenever an acidic compound dissolves in water:
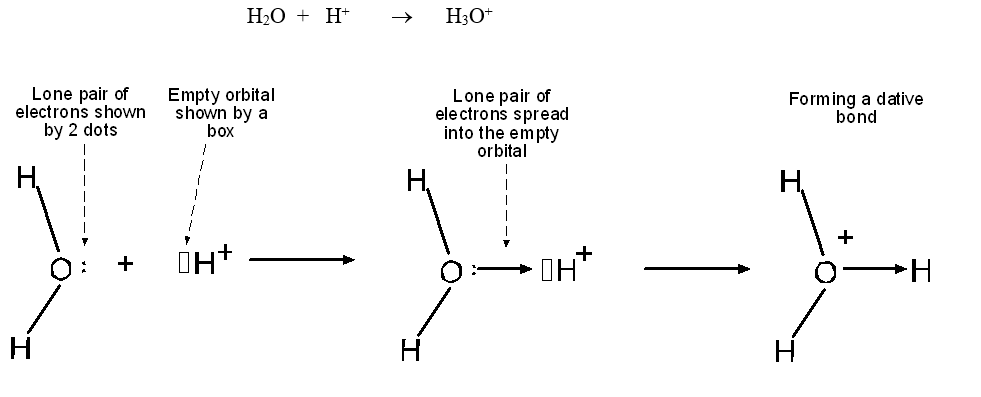
Double and Triple Covalent Bonds.
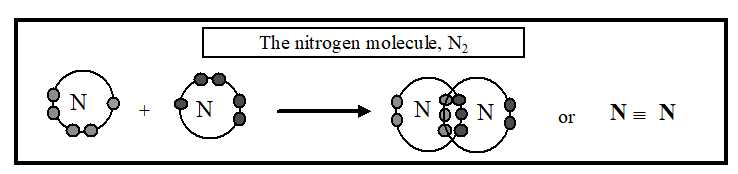
Covalent bonding is not limited to sharing one pair of electrons. In the nitrogen molecule, N2, each nitrogen atom is 3 electrons short of a full shell. Both atoms can fill their outer shells by sharing 3 pairs of electrons, forming a triple bond:
Similarly, in the oxygen molecule, O2, the oxygen atoms are joined by a double bond; O = O. Lewis Diagrams (which show all the valency electrons) are often used to depict the bonds in a molecule. The following are all Lewis Diagrams of the oxygen molecule (note that O = O is not a Lewis Diagram).
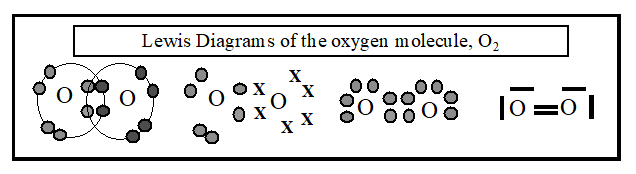
The shape of the molecular orbital depends on which types of atomic orbital overlap to form the covalent bond. We can simplify the possible combinations by grouping them into two types, called sigma bonds and pi bonds.
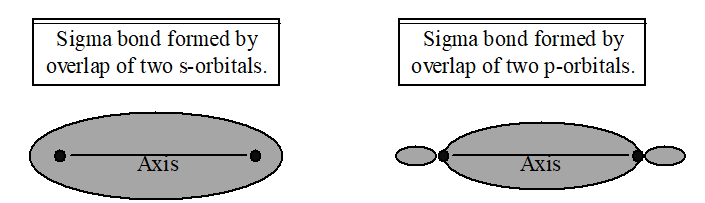
Sigma (s ) Bonds
This is the simplest form of covalent bond; it is a single covalent bond. It is formed when s-orbitals or p-orbitals overlap along the orbital axis; i.e., there is end-on overlap.
Pi (p) Bonds
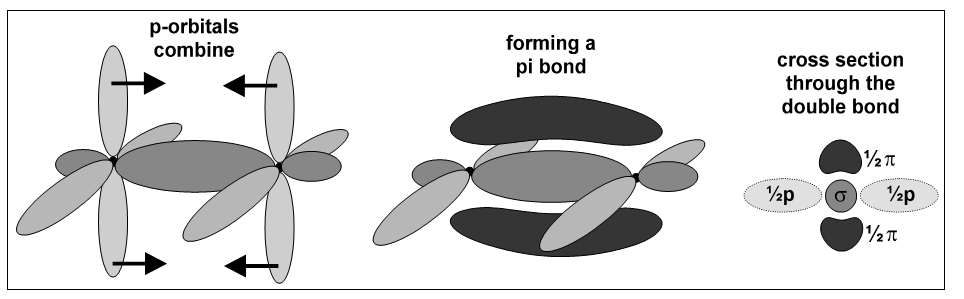
These are considerably more complex than s bonds, and are present in all double bonds. In a double bond, two covalent bonds are formed by the overlap of two half-filled p-orbitals; the first of these is an ordinary sigma bond:
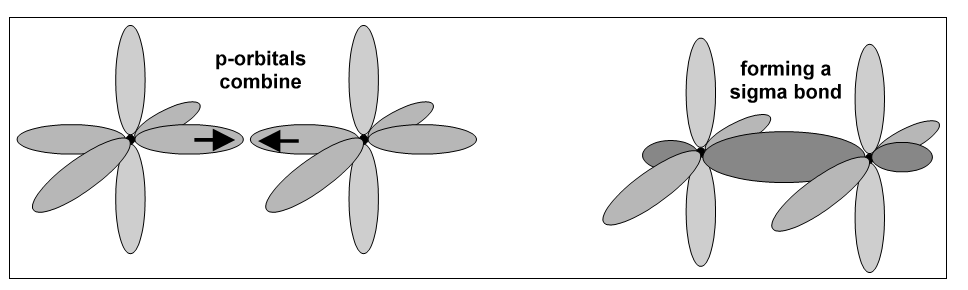
The electrons in the other half-filled p-orbitals can now move from the orbital of one atom to the parallel p-orbital on the other atom. This overlap produces a second bond that is made of two separate regions which are on opposite sides of the s bond. This second bond is a p-bond,
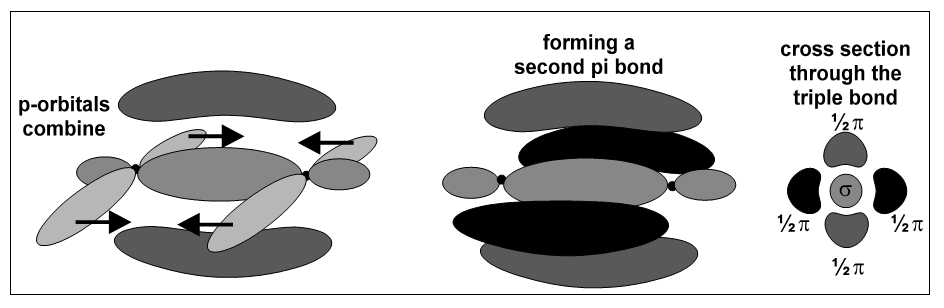
If the remaining p-orbitals are half-filled, this process can be repeated, producing a triple-bond which contains one sigma bond and two pi bonds:
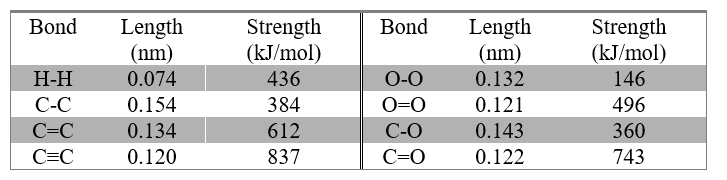
The Shorter, the Stronger.
Everyone knows that it is easier to break a long stick than a short stick. The same is true of chemical bonds. The strength of a covalent bond is called Bond Strength and the distance between two bonded atoms is called Bond Length. Bond strength is defined as “the energy needed to break the bond”, and is measured in kJ.mol-1. In the past, bond length was measured in ångstrom units (Å) (1Å = 10-10 m), but the correct SI unit is the nanometre (nm) (1nm = 10-9 m). Bond lengths and bond strengths vary according to the type of bond and the atoms being joined. The general pattern is that the closer together two atoms are, the harder it is to separate them, so the stronger the bond. The following table gives a sample of bond lengths and strengths.