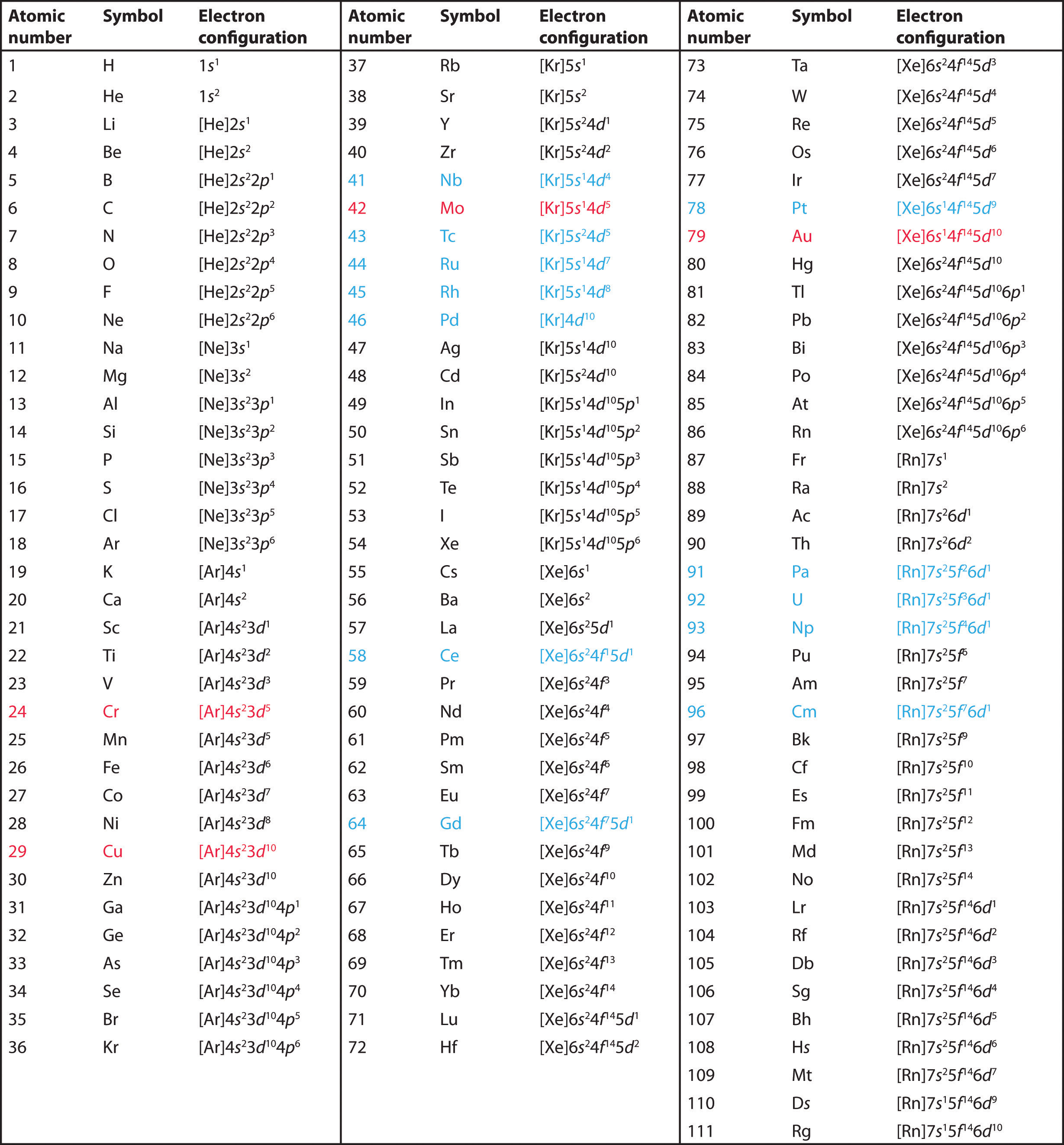
12.1 Electrons in Atoms (AHL)
Essential Idea:
The quantized nature of energy transitions is related to the energy states of electrons in atoms and molecules.
Understandings:
- In an emission spectrum, the limit of convergence at higher frequency corresponds to the first ionization energy
- Trends in first ionization energy across periods account for the existence of main energy levels and sub-levels in atoms.
- Successive ionization energy data for an element give information that shows relations to electron configurations.
Applications and Skills:
- Solving problems using \(E=h\nu \)
- Calculation of the value of the first ionization energy from spectral data which gives the wavelength or frequency of the convergence limit.
- Deduction of the group of an element from its successive ionization energy data.
- Explanation of the trends and discontinuities in first ionization energy across a period.
Calculations of energies
As seen in the SL section all electromagnetic radiation transfers energy in fixed or discrete amounts that are called photons (parcels of energy).
The frequency, v, of electromagnetic waves and the energy, E, of the photons are connected by an equation introduced by Planck:
\(E=h\nu\)
E = energy (of photon) (in joules)
h = Planck’s constant (6.626 x 10-34 J s)
v = frequency (in s-1)
In the case of wave information being giving in terms of wavelength instead of frequency than the above equation needs to be modified.
As \(c=\lambda \nu \), \(\nu\) in Plank’s equation can be substituted by \(\frac{1}{\lambda }\); the equation becomes \(E=\frac{hc}{\lambda }\)
Remember also that some energy values are expressed in joules per mole; this means that any energy value might need to multiplied or divided by 6.02 x 1023.
First ionization energy (IE1)
The first ionization energy of an atom is the minimum energy needed to remove one electron from 1 mole of gaseous atoms (in their ground state) to make gaseous cations as shown by the equation below:
\(X(g)\rightarrow X^+(g)+e^-\)
If an electron in an atom obtains enough energy it jumps or transitions beyond the highest energy level and becomes a free electron; the atom itself becomes a positive ion.
In the hydrogen atom this electron transition is represented by n = 1 to n = ¥. The notation n = ¥ refers to the highest possible energy level in an atom an electron can be on. As once on this level, an electron only needs a very minute amount of energy to leave the atom and form a positive ion, n= ¥ is also considered the level at which ionization occurs. it refers to the “world” outside the atom – beyond the control of the nucleus; any electron outside an atom has no potential energy (energy = 0).
The first electron that is removed in any atom in its ground state is the electron on the highest energy level in the atom with the atom in its ground state; this electron needs the least amount of energy.
In the case of hydrogen as there is only 1 electron, the removed electron is the electron that is on the first energy level, n=1. The frequency of the emission of the electron transition from n=¥ to n=1 should provide is with first ionization energy. This frequency can be obtained from the convergence limit in the Lyman series as this limit represents n = ¥. The convergence limit or series limit is the point at the end of the emission spectrum were all the lines are next to each other or converged; the frequency at the limit is the ionization energy and can be determined using the spectral data and process below. The process involves determining the frequency at which there is no difference between the frequencies emitted the spectrum i.e. the series limit.
Determining the (first) ionization energy in hydrogen
The frequencies in the table below are the frequencies of radiation emitted during electron transitions between different energy levels and n=1 or the ground state. These frequencies result in lines in the ultraviolet spectrum.
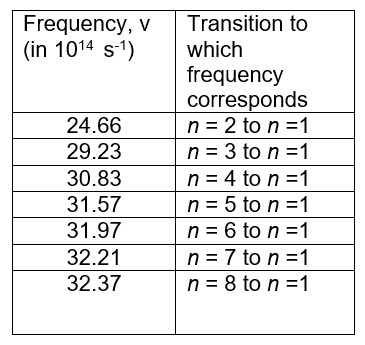
- Work out the difference in frequency, \(\Delta \nu \), between successive lines in the Lyman series for hydrogen
- Plot a graph of \(\Delta \nu \) against frequency – use the value of the lower frequency for plotting \(\nu \)
- Use your graph to estimate the frequency when \(\Delta \nu \) becomes 0.
- \(\Delta \nu \) becomes 0 when the difference in energy between the electronic energy levels becomes 0. Use the relationship E=hv to find the energy which corresponds to the frequency when \(\Delta \nu \) becomes 0. This energy is the first ionization energy for hydrogen but this is usually expressed in kJ mol-1.
The first ionization energy is the energy needed to transition a hydrogen’s electron in an atom in the ground state from n = 2 to n = ∞.
More about the first ionization energy (IE1):
- the more strongly the electron is attracted to the nucleus, the greater the amount of energy needed
- ionization energy is usually measured in kilojoules per mole (kJ mol-1) of atoms;
- ionization energies are positive values (endothermic process) as energy is needed to remove an electron.
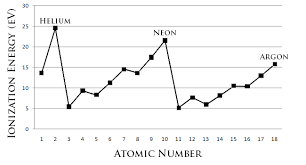
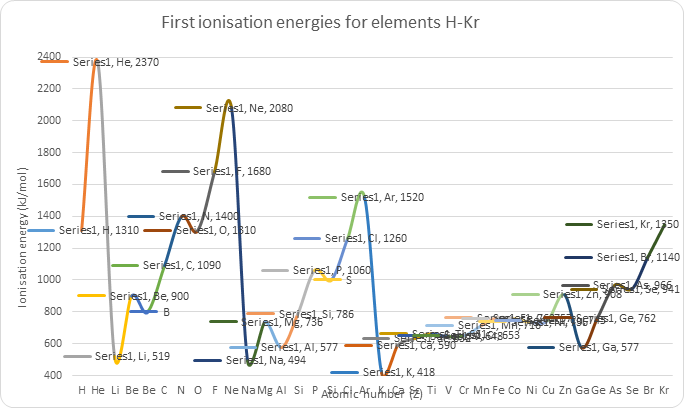
First ionization energies: evidence for main energy levels, sub-levels and Hund’s rule
Observation | evidence for: |
There is a repeated pattern (=periodic trend) of highest values for noble gases followed by a large drop for alkali metals. Peaks of noble gases/troughs of alkali metals also get lower as atomic number increases. | Existence for main energy levels: The large drop is caused by the most loosely held electron being on an energy level much further from nucleus. Peaks of noble gases/troughs of alkali metals also get lower as the most outer electron is on a higher main energy level – same periodic trend but at lower energy because the trend occurs at a higher energy level in the atom. |
Overall increase in first ionization energies across a period. | Existence of main energy level: Electrons are added to same main energy level but nuclear charge increases – greater attraction – more energy needed. |
Decrease in ionization energy between He and Li (and Ne and Na; Ar and K and so on) | Existence of main energy level: The large drop from He to Li can be explained in that the 3rd Li electron (the electron in the highest energy level) is at a higher main energy level than the 2nd electron (the electron in the highest energy level) in He; any large decrease in ionization energy means that the electron is in a higher energy level/less strongly attracted/further away from the nucleus. |
There is no continuous increase between Li and Ne (this period of 8 elements is made up of smaller groups of 2, 3 and 3) | Existence of sub-level: If all the valence electrons of the elements in the second period were in the same main energy level (n=2), a normal steady rise in first ionisation energy would be expected. This is because the electrons would be attracted more strongly by the greater nuclear charge and decrease in atomic radius. However, instead there are 2 drops in ionisation energy between Li and Ne: not all of the most outer electrons of the 8 elements in that period 2 are the same; they are not on the same energy level. |
A decrease from Be to B; this decrease occurs after the second element in the period | Existence of sub-level: · The drop from Be to B indicates that the 5th B electron is at a higher energy level than the 4th Be electron both of which should be on the 2nd main energy level; this has been explained by the fact that the 5th B electron is on a higher sub-level within the 2nd main energy level; · The lowest level at this main energy level is full after 2 electrons. |
There are 6 elements between Be and Ne (Ne including) after which there is again a great decrease | The second level in this main energy level can hold 6 electrons |
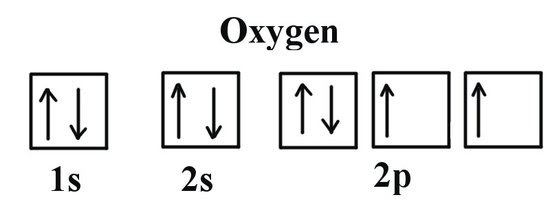
A decrease from N to O (also P to S) | Existence of sub-level: The electron arrangement ensures that the most outer electron in oxygen is at a higher energy level than the most outer electron in nitrogen. This is the case because the electron removed in O is a paired electron (two electrons in same orbital) and this causes greater repulsion and less attraction – Hunds rule. |
Successive ionization energies
- Energy needed to remove second, third,… electrons from 1 mole of gaseous ions, g.
second ionization energy (IE2) \(X^+(g)\rightarrow X^{2+}(g) +e^-\)
third ionization energy (IE3): \( X^{2+}(g)\rightarrow X^{3+}(g) +e^-\)
- The lower the energy level an electron is on, the closer it is to the nucleus, the more strongly it is attracted, the more ionization energy is needed;
- In the same atom, successive ionization energies of electrons in the same energy level increase because mainly the following reasons:
- the electron-electron repulsion decreases (this repulsion puts electrons onto higher energy levels), electrons attracted more inwards and this increases the attraction from the nucleus.
- nucleus has greater effect on smaller number of electrons and they are held more tightly.
- lower shielding effect from fewer inner electrons.
- the first electron is removed from an atom whilst the second and other successive electrons are removed from ions with an increasing positive charge; this attracts the electrons more strongly
- Successive ionization also increases if the next electron is on a lower energy level/closer to the nucleus.
Successive ionization energies: evidence for the main energy levels and the number of electrons that can occupy an energy level.
All the successive ionization energies for the same element can be shown on a graph of successive ionization energies against electrons removed. The successive ionization
Observations of trends in successive ionization energies of aluminium | Explanation: |
3 electrons (3s2 and 3p1; have a much smaller ionization energy than the other remaining electrons which means they need a lot less energy to escape to n = ∞ | These electrons must be on a much higher energy level than the other 10 electrons in the atom; most of the time they are the furthest away from the nucleus and held less strongly. |
· 8 electrons (in 2p and 2s) require a lot more energy than the 3 electrons at the higher energy levels – there is a big jump between the three and these 8 electrons. · There is a gradual increase in I.E. in the 8 electrons | · There are 8 electrons in the same lower main energy level closer to the nucleus. · There is an increase in I.E. as electrons are removed from an increasingly positively charged ion. |
2 electrons (in 1s) need an even higher amount of ionization energy | There are 2 electrons on an even lower energy level; most of the time they are closer to the nucleus than the other electrons and are held very tightly. |
12.1.1 Explain how evidence from first ionization energies across periods accounts for the existence of main energy levels and sub-levels in atoms.
Ionizing energy is how much energy is required to remove one mole of electrons from the ground state of one mole of the gaseous atom. Basically, the energy required to remove an electron from an atom.
As you can see the graph isn’t smooth but it shows a lot of important data. All noble gases which have a full outer shell takes a lot of energy to remove an electron while it is easy for all the alkali metals. It also shows the existence of main energy levels and shows hints of sub-levels.
12.1.2 Explain how successive ionization energy data is related to the electron configuration of an atom
From this graph we could tell that there are certainly 2, 8, 8. But in between there are some anomalies which are explained by the existence of p orbitals.
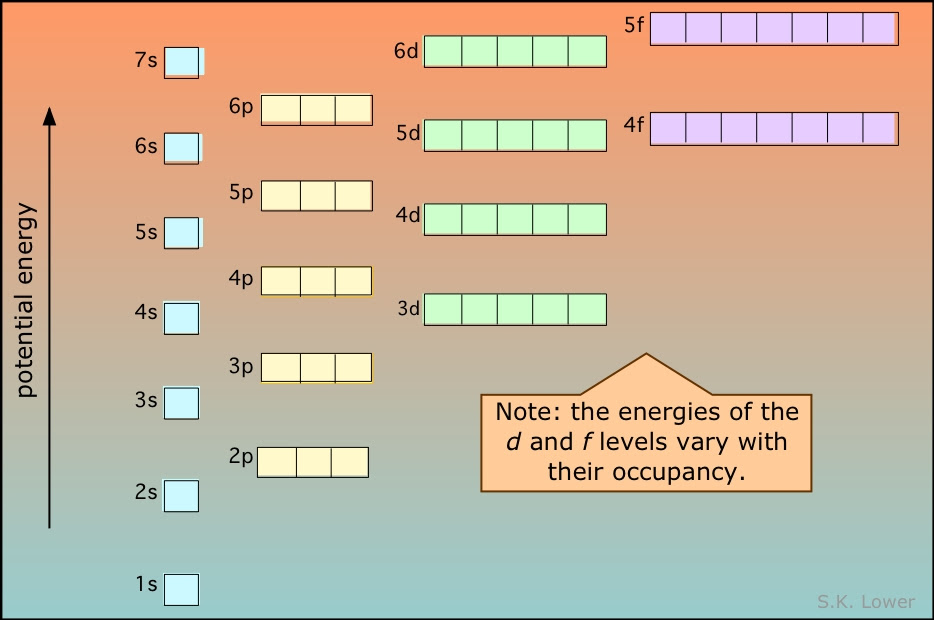
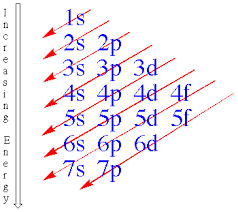
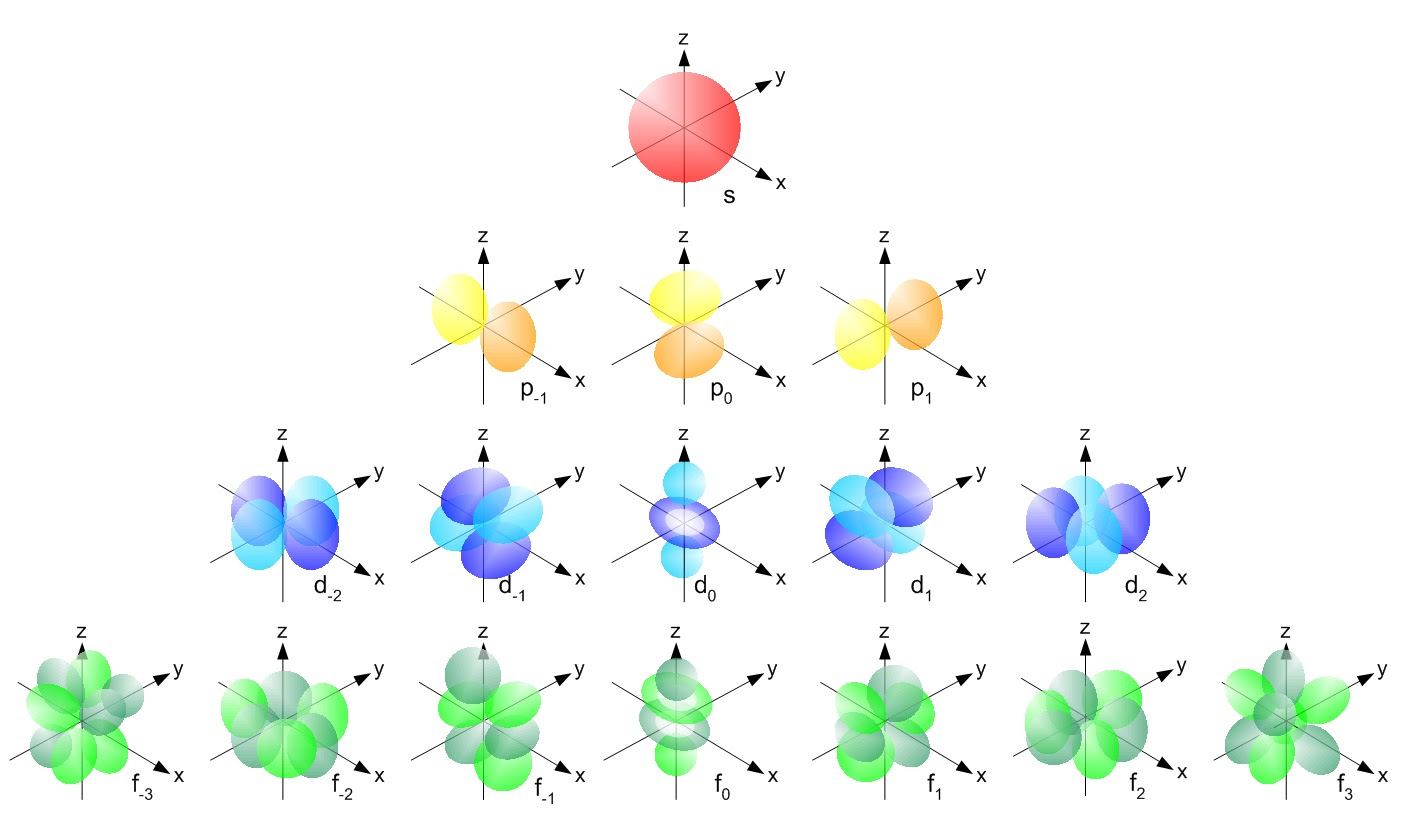
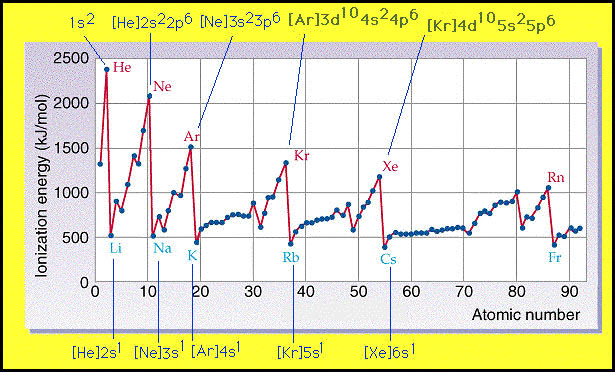
12.1.3 State the relative energy levels of s, p, d and f orbitals in a single energy level.
s is closest to the nucleus, then p, then d and finally f.
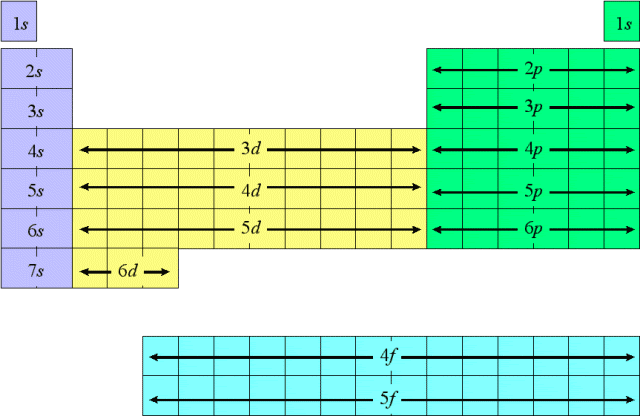
Note: you should be able to tell from the image that 4s actually has a lower energy level than 3d which is the reason why we have transition metals. Similar to 6s and 4f.
The lines show the order of energy. However the number shows the energy level they are in.
12.1.4 State the maximum number of orbitals in a given energy level.
s sub-levels can hold a maximum of 2 electrons
p sub-level can hold a maximum of 6 electrons
d sub-level can hold a maximum of 10 electrons
f sub-level can hold a maximum of 14 electrons.
This shows how the common misconception of 2, 8, 8, 2 electron shells as it is really 1s, 2s 2p, 3s 3p, 4s.
This is how the modern day periodic table is shaped.
12.1.5 Draw the shapes of an s orbital and the shapes of the px, py and pz orbitals.
Simply learn how to draw the first 4 orbitals. The rest is just used to frighten you, they are the shapes of the d and f orbitals.
12.1.6 Apply the Aufbau principle, Hund’s rule and Pauli exclusion principle to write electron configurations for atoms and ions up to Z = 54
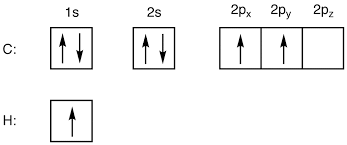
Aufbau principle states that electrons are placed into orbitals of lowest energies first. Boxes can be used to represent the atomic orbitals with single-headed arrows to represent the spinning electrons.
Hund’s rule states that we place them in separate orbitals because this configuration minimizes the mutual repulsion between them.
Pauli Exclusion Principle states that no more than two electrons can occupy any one orbital and if two electrons are in the same orbital they must spin in opposite directions.
Atoms
This is carbon’s electron configuration compared with oxygen and hydrogen.
This is how you draw the electron configuration. There is another method which you write out the configuration by stating all the electrons in each orbital.
Note that you have to know the anomalies to the pattern which is 24 chromium and 29 copper. You only have to learn up to 54. You can also use the short hand form which is putting the noble gas before.
Ions
It is very simple, the electrons of the ion is how many electrons are in the atom and the charge of the ion.
Just in case anyone doesn’t know, you remove the electrons in the same order as you add electrons. However, remember that you remove the electrons in s orbital before the d orbitals.