Question
In which state does 1 \(dm^{3}\) of methane contain the most particles?
A. gas at \(100^{\circ}\)C
B. gas at room temperature
C. liquid
D. solid
▶️Answer/Explanation
Ans:
D
In general, the solid form of a substance has the highest mass compared to the liquid and gas forms. This is because solids are denser and have their particles closely packed together, resulting in a higher mass per unit volume.
Thus, for same volume of substance, methane has highest mass in solid state. Means highest number of moles and hence, highest number of particles.
Question
Nitrogen is heated in a balloon, which expands slightly.
Which statements about the molecules of nitrogen are correct?
1 They move further apart.
2 They move more quickly.
3 They remain the same distance apart.
4 Their speed remains unchanged.
A 1 and 2
B 1 and 4
C 2 and 3
D 3 and 4
▶️Answer/Explanation
Ans:A
When nitrogen gas (N2) is heated inside a balloon, it causes the gas to expand. The expansion occurs because heating the gas increases the average kinetic energy of the nitrogen molecules, making them move more vigorously and collide with the walls of the balloon more frequently and with greater force.
Thus, molecules of nitrogen move more quickly at increased speed and further apart.
Question
What happens to the average speed of gas particles when pressure and temperature are increased?

▶️Answer/Explanation
Ans:D
According to the kinetic theory of gases, the average kinetic energy of gas particles is directly proportional to the temperature of the gas. When the temperature is increased, the gas particles gain more kinetic energy. As a result, they move faster and have higher average speeds.
When pressure is increased while temperature remains constant, the average speed of gas particles does not change.
Question
In which changes do the particles move further apart?
A W and X B W and Z C X and Y D Y and Z
▶️Answer/Explanation
Ans: D
When a substance undergoes a state change from a solid to a liquid or from a liquid to a gas, the particles move further apart.
In the solid state, particles are typically tightly packed in an organized, regular pattern. As the substance is heated or the energy is added, it transitions to the liquid state. In the liquid state, the particles have more freedom of movement, and they can slide past each other while maintaining some level of cohesion.
Further heating or the addition of energy can cause the substance to transition from a liquid to a gas. In the gas state, the particles are widely dispersed and move independently of one another. They have the highest level of freedom of movement and are spaced relatively far apart compared to the solid or liquid state.
Question
Which row describes the arrangement and movement of particles in a liquid?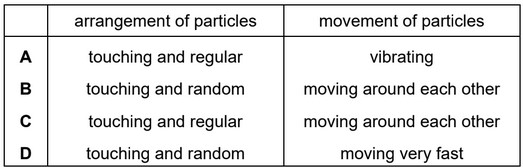
▶️Answer/Explanation
Ans: B
In a liquid, the particles are loosely packed and have more freedom of movement compared to a solid. The arrangement of particles in a liquid is relatively disordered, lacking the rigid, repeating structure found in solids. The particles in a liquid are still attracted to one another, allowing for some level of cohesion and surface tension.
The movement of particles in a liquid is characterized by constant motion. The particles are in continuous motion, vibrating, rotating, and translating. They move around each other, sliding past one another in a random and chaotic manner. The kinetic energy of the particles in a liquid is sufficient to overcome the attractive forces between them, allowing for this fluidity.