Question
‘The movement of a substance very slowly from an area of high concentration to an area of low concentration.’
Which process is being described?
A a liquid being frozen
B a solid melting
C a substance diffusing through a liquid
D a substance diffusing through the air
▶️Answer/Explanation
Ans:C
The process of a substance diffusing through a liquid refers to the movement of particles or molecules from an area of higher concentration to an area of lower concentration within the liquid medium. Diffusion occurs due to the random motion of particles and is driven by the goal of achieving equilibrium, where the concentration of the substance becomes uniform throughout the liquid.
Question
Gases are separated from liquid air by fractional distillation.
The boiling points of four gases are shown.
Which gas is both monoatomic and a liquid at \(–200^o\) C?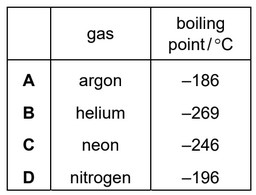
▶️Answer/Explanation
Ans: A
As boiling point of Ar is \(–186^o\) C, which is higher than \(–200^o\) C. It will still be liquid. Ar is monoatomic.
Nitrogen is diatomic.
Question
Decane has a freezing point of –30 °C and a boiling point of 174 °C.
A small sample of decane is placed in an open beaker in an oven at a temperature of 120 °C and
at atmospheric pressure for 24 hours.
What happens to the sample of decane?
A It boils.
B It evaporates.
C It melts.
D It sublimes.
▶️Answer/Explanation
Ans: B
Since the temperature is above freezing point and below boiling point, sample of decane will evaporate. As at temperatures below the boiling point, individual molecules at the liquid’s surface can still gain enough energy to escape into the gas phase. This process is known as evaporation.
Question
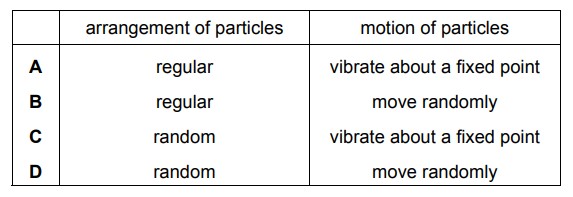
Sodium chloride is a liquid at 900 °C. How are the particles arranged and how do the particles move in sodium chloride at 900 °C?
▶️Answer/Explanation
Ans:
D
The movement of particles in a liquid is characterized by constant motion. The particles are in continuous motion, vibrating, rotating, and translating. They move around each other, sliding past one another in a random and chaotic manner.
Question
The diagram shows a cup of hot tea.

Which row describes the water particles in the air above the cup compared with the water particles in the cup?

▶️Answer/Explanation
Ans:
A
Water particles in the air above the cup have evaporated and are in vapour phase now, hence are moving faster than the water particles in the cup.
Water particles in the air above the cup have evaporated and are in vapour phase now, hence have moved farther than each other than the water particles in the cup which are closer to each other since they are in liquid state.