Question
Which row identifies uses of sulfur?
▶️Answer/Explanation
Ans:
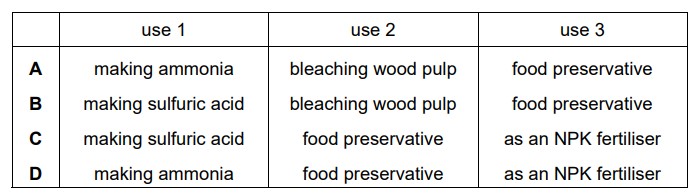
B
Sulfur is a critical raw material for producing sulfuric acid, one of the most widely used industrial chemicals globally.
Sulfur is used in the pulp and paper industry to bleach wood pulp and improve the brightness and quality of paper products. Elemental sulfur or sulfur dioxide is employed in the bleaching process, converting lignin in wood pulp into soluble derivatives, leaving behind white cellulose fibers.
Sulfur is an essential element in the formulation of NPK (Nitrogen, Phosphorus, Potassium) fertilizers. Sulfur-containing fertilizers, such as ammonium sulfate and potassium sulfate, provide a valuable source of sulfur for plants. Sulfur is crucial for plant growth and is an important component of certain amino acids and enzymes necessary for protein synthesis.
Question
Which mixture contains all of the elements in a typical fertiliser?
A ammonium nitrate and calcium phosphate
B ammonium phosphate and potassium chloride
C potassium nitrate and ammonium chloride
D potassium carbonate and ammonium nitrate
▶️Answer/Explanation
Ans:B
A complete fertilizer typically contains three primary nutrients: nitrogen (N), phosphorus (P), and potassium (K), often referred to as NPK. These three elements are essential for plant growth and development. Ammonium phosphate contributes nitrogen and phosphorus, and potassium chloride contributes potassium.
Question
A student writes three statements about potassium nitrate, KNO3.
1 The relative formula mass of KNO3 is 101.
2 Potassium nitrate contains the three essential elements for plant growth.
3 Potassium nitrate could be used as a fertiliser.
Which statements are correct?
A 1 and 2 only B 1 and 3 only C 2 and 3 only D 1, 2 and 3
▶️Answer/Explanation
Ans: B
The relative formula mass of KNO3 (Potassium Nitrate) can be calculated by adding the atomic masses of its constituent elements: potassium (K), nitrogen (N), and oxygen (O).
Relative formula mass of KNO3 = (39.10 + 14.01 + (3 * 16.00)) g/mol ≈ 101 g/mol.
A complete fertilizer typically contains three primary nutrients: nitrogen (N), phosphorus (P), and potassium (K), often referred to as NPK. These three elements are essential for plant growth and development. Potassium nitrate, KNO3 does not contain P.
Potassium nitrate can be used as a fertilizer. It provides a source of potassium and nitrogen to plants, which are essential nutrients for healthy plant growth. Fertilizers containing potassium nitrate are commonly used in agriculture and gardening to supply these nutrients to crops and plants.
Question
Which combination of chemical compounds can be used to produce the fertilizer shown?
A \((NH_4)_3PO_4, KCl\)
B \(NH_4NO_3, Ca_3(PO_4)_2\)
C \(NH_4NO_3, CO(NH_2)_2\)
D \(NH_4NO_3, K_2SO_4, (NH_4)2SO_4\)
▶️Answer/Explanation
Ans: A
\((NH_4)_3PO_4, KCl\) can be combined to produce a fertilizer containing N,P and K.
Question
Which statement about fertilisers is correct?
A Ammonium sulfate, \((NH_4)_2SO_4\), is a better fertiliser than ammonium nitrate, \(NH_4NO_3\), because it contains more oxygen.
B Ammonium phosphate, \((NH_4)_3PO_4\), is a good fertiliser because it contains hydrogen.
C Potassium nitrate, \(KNO_3\), is a good fertiliser because it provides potassium and nitrogen.
D Urea, \((NH_2)_2CO\), is a good fertiliser because it contains carbon.
▶️Answer/Explanation
Ans: C
Potassium nitrate can be used as a fertilizer. It provides a source of potassium and nitrogen to plants, which are essential nutrients for healthy plant growth. Fertilizers containing potassium nitrate are commonly used in agriculture and gardening to supply these nutrients to crops and plants.