Question
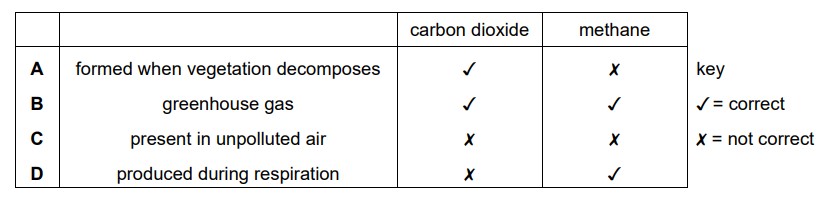
Which information about carbon dioxide and methane is correct?
▶️Answer/Explanation
Ans:
B
Both carbon dioxide and methane are greenhouse gases. Greenhouse gases are gases in the Earth’s atmosphere that trap heat and contribute to the greenhouse effect, which is essential for maintaining a habitable temperature on our planet.
Question
The list shows four methods that were suggested for the formation of carbon dioxide.
1 cracking methane using steam
2 action of heat on a carbonate
3 complete combustion of methane
4 reaction of a carbonate with oxygen
Which methods would result in the production of carbon dioxide?
A 1 and 2
B 1 and 4
C 2 and 3
D 3 and 4
▶️Answer/Explanation
Ans:C
Action of heat on a carbonate: When a carbonate compound (e.g., calcium carbonate) is heated, it undergoes a chemical reaction known as thermal decomposition, resulting in the production of carbon dioxide gas and the corresponding metal oxide.
Complete combustion of methane: When methane, which is the primary component of natural gas, undergoes complete combustion in the presence of oxygen, it produces carbon dioxide and water vapor.
Question
Some gases are present in clean air while other gases are only present in polluted air. Which row is correct?

▶️Answer/Explanation
Ans:B
Argon is an inert, noble gas that makes up a small fraction of the Earth’s atmosphere. It is naturally present in clean air and is not directly influenced by pollution. Argon comprises approximately 0.93% of the Earth’s atmosphere and is the third most abundant gas after nitrogen and oxygen.
Nitrogen dioxide is a reddish-brown gas that is formed when nitrogen oxides (NOx) react with the atmosphere. While some nitrogen oxides are produced naturally from lightning and volcanic activity, human activities are the primary source of NOx emissions. These emissions come from burning fossil fuels in vehicles, power plants, and industrial processes. Therefore, high concentrations of nitrogen dioxide are often associated with urban areas and places with heavy traffic or industrial activities.
Question
Which two gases make up approximately 99% of clean, dry air?
A carbon dioxide and nitrogen
B carbon dioxide and oxygen
C nitrogen and oxygen
D argon and nitrogen
▶️Answer/Explanation
Ans: C
Approximately 78% of the atmosphere consists of nitrogen gas. N2 is a non-reactive, inert gas and plays a crucial role in maintaining the balance of the atmosphere.
Oxygen: Oxygen makes up about 21% of the atmosphere. It is essential for respiration and combustion processes and is vital for supporting aerobic life forms.
Together, nitrogen and oxygen account for around 99% of the atmosphere’s composition.
Question
Which row describes the uses of sulfur and sulfur dioxide?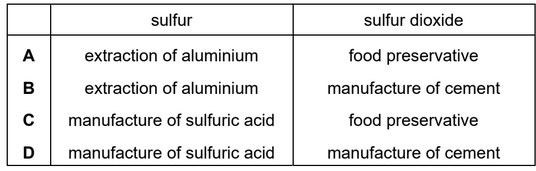
▶️Answer/Explanation
Ans: C
In the manufacture of sulfuric acid, sulfur is the primary raw material used to produce the acid.
Sulfur dioxide is used as a food preservative and antioxidant in certain food products. It has been used for centuries to preserve food because of its antimicrobial and antioxidant properties. However, its use is regulated in many countries due to potential health concerns, especially for individuals who may be sensitive to sulfites.