Question
Polymers are long-chain molecules made from small molecules linked together. Four polymers or types of polymer are listed.
1 carbohydrates
2 nylon
3 proteins
4 Terylene
Which polymers or types of polymer are synthetic?
A. 1 and 3 B. 1 and 4 C. 2 and 3 D. 2 and 4
Answer/Explanation
Ans:
D
Question
Which polymers or types of polymer are synthetic?
1 carbohydrates
2 nylon
3 proteins
4 Terylene
A 1 and 3
B 1 and 4
C 2 and 3
D 2 and 4
▶️Answer/Explanation
Ans:D
Question
Molecule 1 undergoes a process to make molecule 2.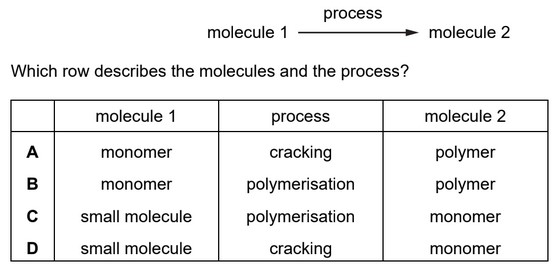
Answer/Explanation
Ans: B
Question
Which substance has long-chain molecules and is a constituent of food?
A carbohydrate
B nylon
C poly(ethene)
D Terylene
Answer/Explanation
Ans: A
Question
Three statements about synthetic polymers are listed.
1 Man-made fibres are used for making clothing.
2 Plastics can cause pollution problems both on land and at sea.
3 Plastics which do not rot away are described as non-biodegradable.
Which statements are correct?
A 1 and 2 only B 1 and 3 only C 2 and 3 only D 1, 2 and 3
Answer/Explanation
Ans: D
Question
Which polymers are natural polymers?
1 carbohydrates
2 poly(ethene)
3 protein
A 1, 2 and 3 B 1 and 3 only C 1 only D 3 only
Answer/Explanation
Ans: B
Question
Which naturally occurring polymers are found in foods?
1 complex carbohydrates
2 nylon
3 salts
4 proteins
A. 1 and 2 B. 1 and 4 C. 2 and 3 D. 3 and 4
Answer/Explanation
Ans:
B
Question
The diagram shows the structure of a monomer and of the polymer made from it.

What are the monomer and polymer?

Answer/Explanation
Ans:
D
Question
Most objects made from synthetic polymers last for many years.
Why do these polymers last for so long?

Answer/Explanation
Ans:
C
Question
Which molecule can be polymerised?

▶️Answer/Explanation
Ans:C
Question
Polymers are long-chain molecules made from small molecules linked together.
Four polymers or types of polymer are listed.
- carbohydrates
- nylon
- proteins
- Terylene
Which of these polymers or types of polymer are synthetic?
A 1 and 3 B 1 and 4 C 2 and 3 D 2 and 4
Answer/Explanation
Ans:
D
Question
Which statement about Terylene is correct?
A It is a form of protein.
B It is a natural polymer.
C It is also called poly(ethene).
D It is used to make clothes.
Answer/Explanation
Ans:D
Question
Which statement about polymers is correct?
- Polymers are formed by breaking down monomers.
- Polymers can be natural or synthetic.
- Polymers contain atoms of only one element.
- Polymers have a giant ionic structure.
Answer/Explanation
Ans:
B
Question:
Molecules of a substance react together as shown.

Which type of reaction has taken place?
A cracking
B oxidation
C polymerisation
D reduction
Answer/Explanation
Ans: C
Question:
The diagram shows the structure of an important product.

This product is formed by ……1.….. of an .….. 2…… .
Which words complete gaps 1 and 2?

Answer/Explanation
Ans: B
Question:
Which type of hydrocarbon reacts rapidly with aqueous bromine and what is the colour change of the aqueous bromine?

Answer/Explanation
Ans: C
Question:
The diagram shows part of the molecule of a polymer.

Which diagram shows the monomer from which this polymer could be manufactured?

Answer/Explanation
Ans: C
Question
Ethene forms an addition polymer as shown.

Which terms describe this polymer?
A a saturated compound called poly(ethane)
B a saturated compound called poly(ethene)
C an unsaturated compound called poly(ethane)
D an unsaturated compound called poly(ethene)
Answer/Explanation
Ans:B
Question
Information about four hydrocarbons is shown.

Which statement is correct?
A Hydrocarbon W has the formula \(CH_4\) and can be polymerised.
B Hydrocarbon X has the formula \(C_2H_4\) and can be polymerised.
C Hydrocarbon Y has the formula \(C_3H_6\) and can be polymerised.
D Hydrocarbon Z has the formula \(C_4H_10\) and can be polymerised.
Answer/Explanation
Ans:C
Question
Which row describes the formation of a polymer?
Answer/Explanation
Ans: D
Question
A hydrocarbon A is cracked to make B and hydrogen.
Compound C is formed by the addition polymerisation of B.
To which homologous series do A, B and C belong?
Answer/Explanation
Ans: B
Question
The diagram shows three repeat units in the structure of an addition polymer.

Which alkene monomer is used to make this polymer?

Answer/Explanation
Ans:
C
Question
A hydrocarbon X is cracked to make Y and hydrogen.
Compound Z is formed by the addition polymerisation of Y.
To which homologous series do X, Y and Z belong?
Answer/Explanation
Ans: C
Question
PVA is a polymer. The monomer has the structure shown.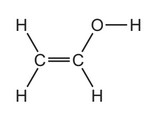
To which homologous series does this compound belong?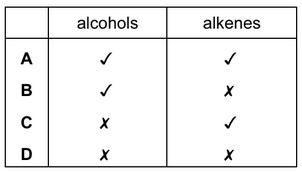
Answer/Explanation
Ans: A