Question
A sign displayed in a flour mill is shown.

Which statement explains why there is a danger of explosion in a flour mill?
A Flour burns very quickly because it is a fine powder.
B Flour is a catalyst for combustion.
C Flour mills get hot and speed up the rate of combustion.
D The combustion of flour is exothermic.
▶️Answer/Explanation
Ans:A
Question
The apparatus shown is used to prepare aqueous copper(II) sulfate.

What are X and Y?

▶️Answer/Explanation
Ans:C
Question
Fermentation of sugar produces a mixture of ethanol solution and solid yeast. How is the solid yeast removed from the mixture?
A. crystallisation
B. distillation
C. filtration
D. fractional distillation
Answer/Explanation
Ans:
C
Question
Which piece of apparatus is used to measure exactly 5.00 \(cm^{3}\) of a liquid?
A. 5 \(cm^{3}\) beaker
B. 10 \(cm^{3}\) measuring cylinder
C. 25 \(cm^{3}\) pipette
D. 50 \(cm^{3}\) burette
Answer/Explanation
Ans:
D
Question
Which piece of apparatus should be used to measure exactly $21.4 \mathrm{~cm}^3$ of water?
A $25 \mathrm{~cm}^3$ beaker
B $25 \mathrm{~cm}^3$ pipette
C $50 \mathrm{~cm}^3$ burette
D $50 \mathrm{~cm}^3$ measuring cylinder
▶️Answer/Explanation
Ans:C
Question
Which piece of apparatus can only measure a single fixed volume?
A $250 \mathrm{~cm}^3$ beaker
B $50 \mathrm{~cm}^3$ burette
C $100 \mathrm{~cm}^3$ measuring cylinder
D $25 \mathrm{~cm}^3$ pipette
▶️Answer/Explanation
Ans:D
Question
Why is sulfur dioxide used as a food preservative?
A It is a gas at room temperature.
B It is used to make sulfuric acid.
C It kills bacteria.
D It reacts with alkalis.
Answer/Explanation
Ans: C
Question
Which statements about lime (calcium oxide) and limestone (calcium carbonate) are correct?
1 Limestone is used in the manufacture of iron.
2 Lime is made by heating limestone.
3 Powdered limestone is heated with clay in the production of cement.
4 Limestone causes soil to be acidic.
A 1 and 2 only B 2 and 3 only C 1, 2 and 3 D 1, 3 and 4
Answer/Explanation
Ans: C
Question
Which piece of apparatus is used to measure 1.5 \(cm^3\) of a solution accurately?
A 25 \(cm^3\) measuring cylinder
B 25 \(cm^3\) pipette
C 50 \(cm^3\) measuring cylinder
D 50 \(cm^3\) burette
Answer/Explanation
Ans: D
Question
The diagram represents a lime kiln used to heat limestone to a very high temperature.

What leaves the kiln at X?
A. calcium carbonate
B. calcium hydroxide
C. calcium oxide
D. calcium sulfate
Answer/Explanation
Ans:
C
Question
The apparatus shown is set up and left for a week.

Which diagram shows the level of the water at the end of the week?
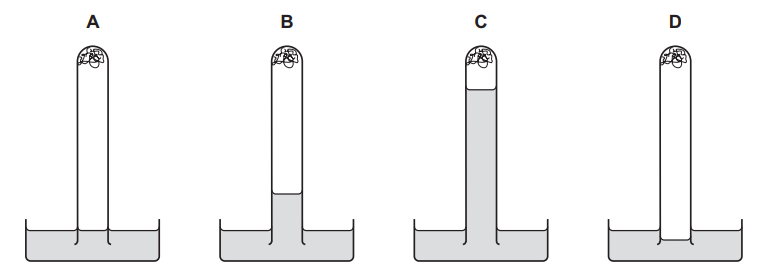
Answer/Explanation
Ans:
B
Question
2.00 g of powdered calcium carbonate is added to 50.0 \(cm^{3}\) of hydrochloric acid. Which apparatus is used to measure the calcium carbonate and the hydrochloric acid?
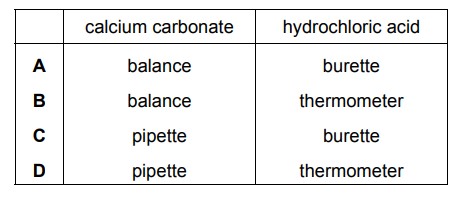
Answer/Explanation
Ans:
A
Question
A fractionating column is used to separate the hydrocarbon fractions in petroleum by fractional distillation.
Which row describes the properties of the fractions that condense at the top of the fractionating column?

Answer/Explanation
Ans:
D
Question
The fractional distillation of petroleum is shown.

Which fraction is the least volatile?
- bitumen
- diesel oil
- gasoline fraction
- refinery gas
Answer/Explanation
Ans:
A
Question
Lead(II) iodide is insoluble in water.
Lead(II) iodide is made by adding aqueous lead(II) nitrate to aqueous potassium iodide.
Which pieces of apparatus are needed to obtain solid lead(II) iodide from 20 cm3 of aqueous lead(II) nitrate?

A 1, 2 and 4 B 1, 3 and 5 C 1, 4 and 5 D 2, 4 and 5
Answer/Explanation
Ans:
C
Question
In the experiment shown, a white precipitate forms in the limewater.
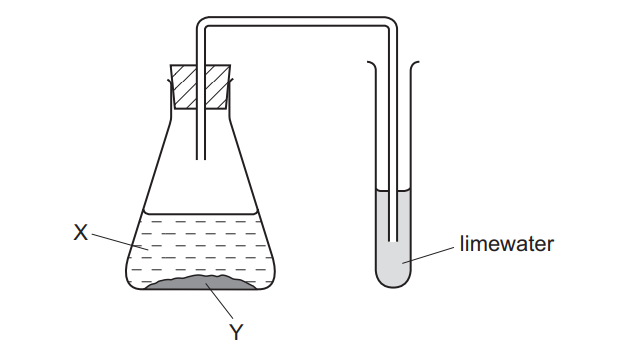
What are X and Y?
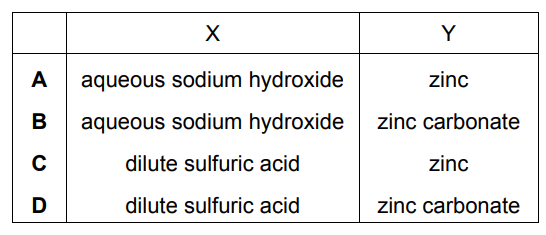
▶️Answer/Explanation
Ans:D
Question
Which box corresponds to limestone?
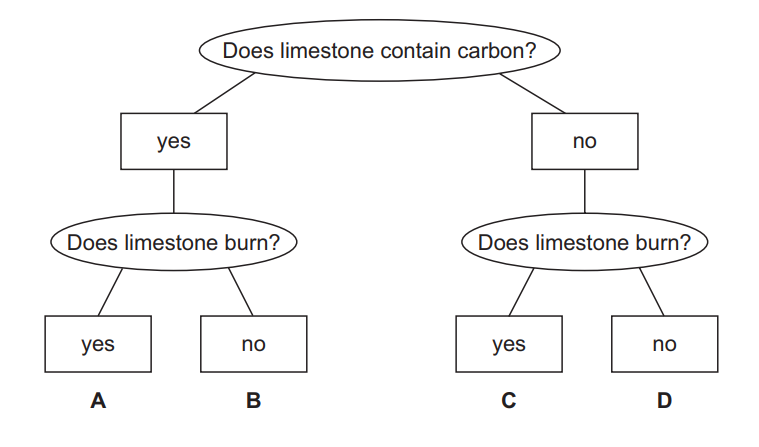
▶️Answer/Explanation
Ans:B
Question
‘Particles moving very slowly from an area of higher concentration to an area of lower concentration.’
Which process is being described?
A a liquid being frozen
B a solid melting
C a substance diffusing through a liquid
D a substance diffusing through the air
▶️Answer/Explanation
Ans:C
Question
Which process is used to convert limestone (calcium carbonate) into lime?
- electrolysis
- fractional distillation
- incomplete combustion
- thermal decomposition
Answer/Explanation
Ans:
D
Question
Dry air is passed over hot copper until all the oxygen has reacted.

The volume of gas at the end of the reaction is 120 cm3.
What is the starting volume of dry air?
A 132 cm3 B 152 cm3 C 180 cm3 D 570 cm3
Answer/Explanation
Ans:
B
Question
The diagrams show four pieces of laboratory equipment.

Which equipment is essential to find out if dissolving a salt in water is an exothermic process?

Answer/Explanation
Ans:D
Question
Which piece of apparatus is used to measure exactly 26.3 cm3 of a liquid?

Answer/Explanation
Ans:
A
Question
Substance L melts at –7°C and is a brown liquid at room temperature.
Which temperature is the boiling point of pure L?
- –77 °C
- –7 °C to +7 °C
- 59 °C
- 107 °C to 117 °C
Answer/Explanation
Ans:
C
Question
The diagram shows liquid in a burette and in a measuring cylinder.

Which row shows the readings for the burette and the measuring cylinder?

Answer/Explanation
Ans:
B
Question:
Two experiments are carried out.
In experiment 1, copper is heated with steam.
In experiment 2, copper(II) oxide is heated with carbon.

Which row describes what happens in experiments 1 and 2?

Answer/Explanation
Ans: B
Question:
A student put 25.0 cm3
of dilute hydrochloric acid into a conical flask.
The student added 2.5 g of solid sodium carbonate and measured the change in temperature of the mixture.
Which apparatus does the student need to use to obtain the most accurate results?
A balance, measuring cylinder, thermometer
B balance, pipette, stopwatch
C balance, pipette, thermometer
D burette, pipette, thermometer
Answer/Explanation
Ans: C
Question:
The diagrams show liquids in a burette and a measuring cylinder.

Which row shows the correct readings for the burette and the measuring cylinder?]

Answer/Explanation
Ans: B
Question:
Which statement is not correct?
A Converting limestone into lime is a thermal decomposition reaction.
B Flue gas desulfurisation is a neutralisation reaction.
C In the extraction of iron, calcium carbonate is converted into calcium oxide.
D Slaked lime is added to soil as a fertiliser.
Answer/Explanation
Ans: D
Question:
The diagram shows part of a thermometer.

What is the reading on the thermometer?
A 30.2 B 30.3 C 31.7 D 31.8
Answer/Explanation
Ans: B
Question
The diagram shows the separation of petroleum into fractions.

What could X, Y and Z represent?

Answer/Explanation
Ans:D
Question
Which statement describes a disadvantage of sulfur dioxide?
A It can be used as a bleach in making wood pulp.
B It can be used to kill bacteria in food.
C It can be used to manufacture sulfuric acid.
D It can dissolve the limestone in statues.
Answer/Explanation
Ans:D
Question
What is the name of fraction X?

A alcohol
B fuel oil
C naphtha
D paraffin
Answer/Explanation
Ans:C
Question
The apparatus shown is set up and left for a week.
Which diagram shows the level of the water at the end of the week?
Answer/Explanation
Ans: B
Question
The diagram represents a lime kiln.
What leaves the furnace at X?
A calcium carbonate
B calcium hydroxide
C calcium oxide
D calcium sulfate
Answer/Explanation
Ans: C
Question
Powdered marble reacts with hydrochloric acid using the apparatus shown.
The gas syringe fills in 36 seconds.
The experiment is repeated using marble chips in place of powdered marble.
How long does it take to fill the gas syringe in this experiment?
A 9 seconds
B 18 seconds
C 36 seconds
D 72 seconds
Answer/Explanation
Ans: D
Question
A student uses the apparatus shown in the diagram below to measure the volume of carbon
dioxide gas made when different masses of marble chips are added to 25\(cm^3\) of dilute hydrochloric acid.
Which other items of apparatus are needed?
A funnel and balance
B funnel and stopwatch
C measuring cylinder and balance
D measuring cylinder and stopwatch
Answer/Explanation
Ans: C
Question
The diagram shows a kiln used to heat limestone.

What is the product and what waste gas is formed?

Answer/Explanation
Ans:
B
Question
The diagram shows an experiment to investigate how paint affects the rusting of iron.
What happens to the water level in tubes P and Q?
Answer/Explanation
Ans: D
Question
Air containing an acidic impurity was neutralised by passing it through a column containing substance X.
What is substance X?
A calcium oxide
B sand
C sodium chloride
D concentrated sulfuric acid
Answer/Explanation
Ans: A