Question
Molten iron from the blast furnace contains impurities.
The process of turning the impure iron into steel involves blowing oxygen into the molten iron and adding calcium oxide.
What are the reasons for blowing in oxygen and adding calcium oxide?

▶️Answer/Explanation
Ans:A
Question
Petroleum is separated into fractions by fractional distillation.
Separation occurs in a fractionating column.
Some properties of three of these fractions are shown.

Which statement is correct?
A Fraction 1 has a higher boiling point range than fraction 2.
B Fraction 2 is removed from a higher point in the fractionating column than fraction 1.
C Molecules in fraction 3 have shorter chains than those in fraction 2 .
D None of the fractions are liquid at room temperature.
▶️Answer/Explanation
Ans:C
Question
Copper(II) sulfate is prepared by adding excess copper(II) oxide to warm dilute sulfuric acid.
Which purification methods are used to obtain pure solid copper(II) sulfate from the reaction
mixture?
1 crystallisation
2 filtration
3 chromatography
4 distillation
A 1 and 4 B 1 and 2 C 2 and 3 D 3 and 4
Answer/Explanation
Ans: B
Question
A mixture is separated using the apparatus shown.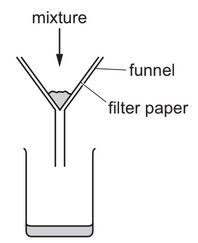
What is the mixture?
A aqueous copper(II) sulfate and aqueous sodium chloride
B aqueous copper(II) sulfate and copper
C copper and sulfur
D ethanol and ethanoic acid
Answer/Explanation
Ans: B
Question
The apparatus used to separate a mixture of sand, methanol and ethanol is shown.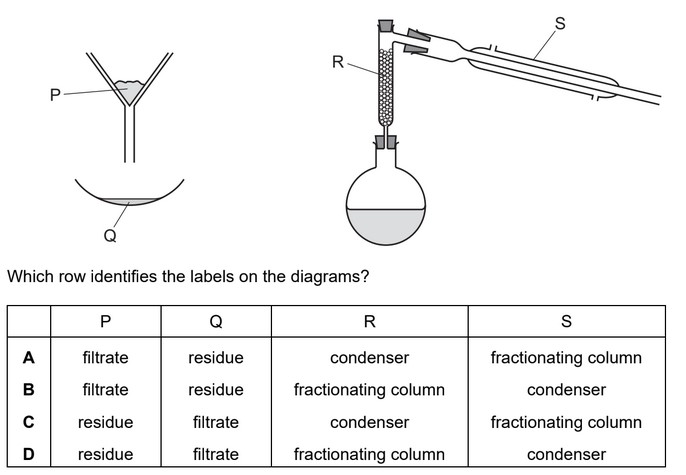
Answer/Explanation
Ans: D
Question
A student separates sugar from pieces of broken glass by dissolving the sugar in water and filtering off the broken glass.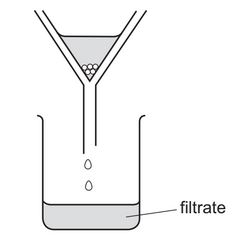
What is the filtrate?
A broken glass only
B broken glass and sugar solution
C pure water
D sugar solution
Answer/Explanation
Ans: D
Question
Copper(II) chloride crystals are made by adding solid copper(II) carbonate to dilute
hydrochloric acid until no more dissolves.
Which process is used to obtain pure copper(II) chloride crystals from the mixture?
A distillation of the mixture
B evaporation of the mixture
C filtration followed by drying of the residue
D filtration followed by evaporation of the filtrate
Answer/Explanation
Ans: D
Question
Which statement about aqueous sodium hydroxide is correct?
A When it is added to a solution containing sulfate ions, a white precipitate is formed.
B When it is added to a solution of copper(II) ions, a blue precipitate is formed which dissolves in excess to give deep blue solution.
C When it is added to a solution of iron(II) ions, a green precipitate is formed which does not dissolve in excess.
D When it is added to ammonium chloride, a gas is produced which turns blue litmus red.
Answer/Explanation
Ans: C
Question
Which process is used to obtain lime from limestone?
- cracking
- fractional distillation
- neutralization
- thermal decomposition
Answer/Explanation
Ans:
D
Question:
Which method can be used to separate a mixture of salt and water to obtain both parts of the mixture?
A crystallisation
B distillation
C evaporation
D filtration
Answer/Explanation
Ans: B
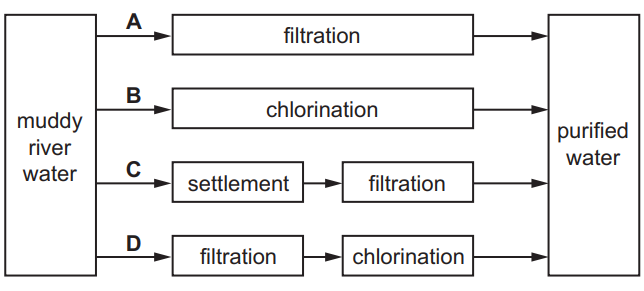
Question:
The diagram shows how muddy water can be purified.

Which process for purifying the muddy water is shown?
A crystallisation
B distillation
C filtration
D solvent extraction
Answer/Explanation
Ans: C
Question
Which method separates a mixture of sugar and glass?
A dissolve, filter and evaporate
B distillation and filter
C fractionally distillation
D use chromatography
Answer/Explanation
Ans:A
Question.
Which method of purification would produce water most suitable for drinking?
Answer/Explanation
Ans:
D
Question
Which statement about methane is not correct?
- It is a liquid produced by distilling petroleum.
- It is produced as vegetation decomposes.
- It is produced by animals, such as cows.
- It is used as a fuel.
Answer/Explanation
Ans:
A
Question
A mixture is separated using the apparatus shown.
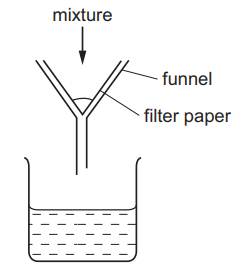
What is the mixture?
- aqueous copper chloride and copper
- aqueous copper chloride and sodium chloride
- ethane and methane
- ethanol and water
Answer/Explanation
Ans:
A
Question
The table shows some fractions that are obtained from petroleum by fractional distillation, together with some of their uses.

Which row correctly identifies fractions 1, 2 and 3?

Answer/Explanation
Ans:
B
Question
Petroleum is a mixture of hydrocarbons which can be separated into fractions using fractional distillation.
Which fraction is used as fuel in jet engines?
- bitumen
- gasoline
- kerosene
- naphtha
Answer/Explanation
Ans:
C
Question
A solid mixture contains an ionic salt, X, and a covalent organic compound, Y.
Two students suggest methods of separating the mixture as shown.
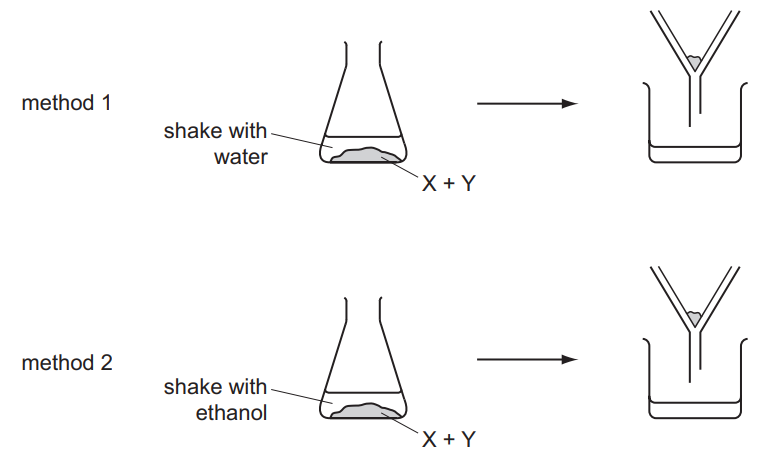
Which methods of separation are likely to work?

Answer/Explanation
Ans:
A
Question
Which properties of the different compounds in petroleum enable its separation into fractions?
1 boiling point
2 chain length
3 chemical reactivity
4 solubility in water
A 1 and 2 B 1 and 3 C 2 and 4 D 3 and 4
Answer/Explanation
Ans: A
Question
Bitumen is a substance obtained from the fractional distillation of petroleum.
Which row describes its boiling point and the size of its molecules?
Answer/Explanation
Ans: A
Question
Pieces of copper, iron, magnesium and zinc are added to separate test-tubes containing dilute hydrochloric acid.
Which test-tube contains iron and dilute hydrochloric acid?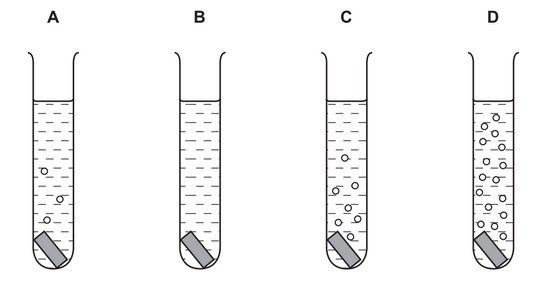
Answer/Explanation
Ans: A
Question
The diagram shows apparatus used to separate petroleum into four fractions.
Which fraction contains the smallest hydrocarbon molecules?
Answer/Explanation
Ans: A
Question
Which fraction from the fractional distillation of petroleum does not match its correct use?
Answer/Explanation
Ans: C