Question
Matter exists as elements, compounds and mixtures. Which row identifies an element, a compound and a mixture?

▶️Answer/Explanation
Ans:
C
Calcium is an element.
Sodium chloride, NaCl is a compound formed from Na and Cl elements.
Brass is a mixture of copper and zinc. It is an alloy, which is a solid solution of two or more metals. Brass typically contains varying proportions of copper and zinc, with the exact composition depending on the desired properties and specific application.
Question
Which substances can be separated by filtration?
A insoluble liquid and water
B insoluble solid and water
C solution of soluble liquid in water
D solution of soluble solid in water
▶️Answer/Explanation
Ans:B
Filtration is an effective method for separating insoluble solids from a liquid, as the filter paper acts as a physical barrier, allowing only the liquid to pass through while retaining the solid particles.
Question
Which diagram represents a mixture of compounds?

▶️Answer/Explanation
Ans:C
A mixture of compounds refers to a combination of two or more different compounds that are physically intermingled but not chemically bonded to each other. In a mixture of compounds, each compound retains its individual chemical identity and properties.
C represents a mixture of compounds as there are two different compounds that are physically intermingled.
Question
Impurities change the melting and boiling points of substances.
Sodium chloride is added to a sample of pure water.
How does the addition of sodium chloride affect the melting point and the boiling point of the
water?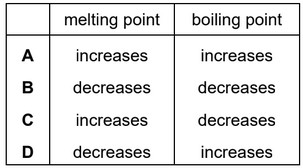
▶️Answer/Explanation
Ans: D
The addition of sodium chloride to water lowers the freezing point of the water, causing it to have a lower melting point. This phenomenon is known as freezing point depression. When sodium chloride is dissolved in water, it dissociates into sodium ions (Na+) and chloride ions (Cl-). These ions disrupt the arrangement of water molecules, making it more difficult for them to form a solid lattice structure during freezing. As a result, the presence of dissolved sodium chloride reduces the attractive forces between water molecules, making it necessary to cool the solution to a lower temperature for it to solidify (freeze). The more sodium chloride is dissolved in water, the lower the freezing point of the solution becomes.
The addition of sodium chloride to water increases the boiling point of the water. This phenomenon is known as boiling point elevation. When sodium chloride is dissolved in water, the sodium and chloride ions interact with the water molecules. These interactions create additional forces that need to be overcome to convert the liquid water into vapor during boiling. As a result, the presence of dissolved sodium chloride increases the boiling point of the solution. The more sodium chloride is dissolved in water, the higher the boiling point of the solution becomes.
Question
A student put exactly 25.00 \(cm^3\) of dilute hydrochloric acid into a conical flask.
The student added 2.5 g of solid sodium carbonate and measured the change in temperature of
the mixture.
Which apparatus does the student need to use?
A balance, measuring cylinder, thermometer
B balance, pipette, stopwatch
C balance, pipette, thermometer
D burette, pipette, thermometer
▶️Answer/Explanation
Ans: C
Balance is needed to measure weight of solid sodium carbonate.
Pipette is needed to measure volume of dilute hydrochloric acid.
Thermometer is needed to measure the initial and final temperatures of the mixture.