Question
The atomic number and nucleon number of a potassium atom are shown.
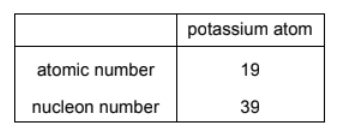
How many protons, neutrons and electrons are in a potassium ion, $\mathrm{K}^{+}$?

▶️Answer/Explanation
Ans:A
The atomic number of potassium (K) is 19, which means it normally has 19 protons and 19 electrons. However, when it loses one electron to form the K+ ion, the number of electrons reduces to 18.
The number of protons remains the same at 19 because it determines the element’s identity.
Number of neutrons = Nucleon number – number of protons
Number of neutrons = 39-19=20.
Question
An atom of element R contains 15 protons, 16 neutrons and 15 electrons. What is R?
A gallium
B phosphorus
C sulfur
D zinc
▶️Answer/Explanation
Ans:B
As it has 15 protons and 15 electrons, its atomic number is 15. It is Phosphorus.
Question
Element X has 7 protons.
Element Y has 8 more protons than X.
Which statement about element Y is correct?
A Y has more electron shells than X.
B Y has more electrons in its outer shell than X.
C Y is in a different group of the Periodic Table from X.
D Y is in the same period of the Periodic Table as X.
▶️Answer/Explanation
Ans: A
Element X has 7 protons. Its atomic number is 7. It is nitrogen.
Element Y has 8 more protons than X. It has 15 protons. It is phosphorus.
Y has more electron shells than X.
The electron configuration of nitrogen (N) is 1s² 2s² 2p³.
The electron configuration of phosphorus (P) is 1s² 2s² 2p⁶ 3s² 3p³.
They are in same group of periodic table. They have same number of electrons in their outer shell.
Question
A neutral atom, J, contains 45 neutrons and 35 electrons.
Which row is correct for atom J?
▶️Answer/Explanation
Ans: B
Number of protons = Number of electrons = 35
Nucleon number = Number of protons + number of neutrons = 35+45=80.
Question
A representation of an atom is shown.
What is the nucleon number of this atom?
A 6 B 7 C 12 D 13
▶️Answer/Explanation
Ans: D
Here, neutrons are shown by shaded circles and protons by + sign.
Number of nucleons = Number of entities present in nucleus = Number of protons + Neutrons = 6+7=13.