Question
Cesium fluoride is an ionic compound. Which statements about cesium fluoride are correct?
1. It conducts electricity when solid.
2. It has a high melting point.
3. It is soluble in water.
4. It is highly volatile.
A. 1 and 2 B. 1 and 4 C. 2 and 3 D. 3 and 4
▶️Answer/Explanation
Ans:
C
Cesium fluoride has a high melting point. Cesium fluoride is highly soluble in water. In the molten state or when dissolved in water, cesium fluoride can conduct electricity.
It has high boiling point. It is very less volatile.
Question
The electronic structures of two atoms, $\mathrm{P}$ and $\mathrm{Q}$, are shown.
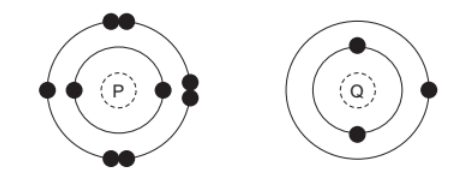
P and Q combine together to form a compound.
What is the type of bonding in the compound and what is the formula of the compound?

▶️Answer/Explanation
Ans:A
P has 7 electrons in outermost shell.
Q has 1 electron in outermost shell.
Q will donate 1 electron to P and form an ionic bond.
PQ will be formed after this ionic bonding.
Question
Which row describes the properties of potassium iodide, KI?

▶️Answer/Explanation
Ans:D
Potassium iodide (KI) is an ionic compound consisting of the potassium cation and the iodide anion.
It has high boiling point.
It does not conduct electricity in solid state. However, when dissolved in water or in a molten state, potassium iodide dissociates into potassium cations and iodide anions, which are mobile and can conduct electricity.
Question
Which row describes how an ionic bond forms between a sodium atom and a chlorine atom?
▶️Answer/Explanation
Ans: D
The electron configuration of sodium is 1s² 2s² 2p⁶ 3s¹, indicating that it has one valence electron in its outermost energy level (3s¹).
The electron configuration of chlorine is 1s² 2s² 2p⁶ 3s² 3p⁵, indicating that it has seven valence electrons in its outermost energy level (3s² 3p⁵).
Sodium has a tendency to lose its single valence electron in order to achieve a stable electron configuration similar to that of a noble gas. Chlorine, on the other hand, has a strong tendency to gain one electron to achieve a stable electron configuration like a noble gas.
The sodium atom donates its single valence electron to the chlorine atom to form NaCl.
Question
Lithium and fluorine react to form lithium fluoride.
A student writes three statements about the reaction.
1 Lithium atoms lose an electron when they react.
2 Each fluoride ion has one more electron than a fluorine atom.
3 Lithium fluoride is a mixture of elements.
Which statements are correct?
A 1 and 2 only B 1 and 3 only C 2 and 3 only D 1, 2 and 3
▶️Answer/Explanation
Ans: A
The electron configuration of lithium is 1s² 2s¹, indicating that it has one valence electron in its outermost energy level (2s¹). Fluorine, with an atomic number of 9, has 9 protons and 9 electrons. Its electron configuration is 1s² 2s² 2p⁵, indicating that it has seven valence electrons (2s² 2p⁵).
Lithium has a strong tendency to lose its single valence electron to achieve a stable electron configuration similar to that of a noble gas (helium). Fluorine has a strong tendency to gain one electron to achieve a stable electron configuration like a noble gas (neon).
The lithium atom donates its single valence electron to the fluorine atom.
The reaction between lithium and fluorine involves the transfer of an electron from lithium to fluorine, resulting in the formation of oppositely charged lithium cations and fluoride anions. These charged ions are then held together by electrostatic attraction, leading to the formation of the ionic compound lithium fluoride.