Question
Which pair of statements about diamond and graphite is correct?
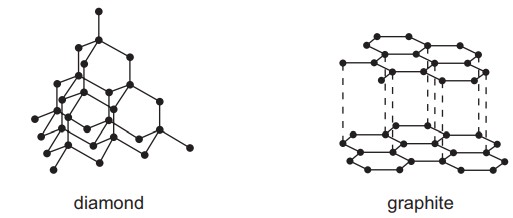
A. Diamond and graphite are both pure carbon. They are both macromolecules.
B. Diamond and graphite can both be used as electrodes. Graphite is also used as a lubricant.
C. Diamond has covalent bonds. Graphite has ionic bonds.
D. Diamond is hard with a high melting point. Graphite is soft with a low melting point.
▶️Answer/Explanation
Ans:
A
Diamond and graphite are both pure carbon. They are both macromolecules.
Graphite is soft and slippery due to the weak forces between the layers. The layers can easily slide past each other, giving graphite its lubricating properties.
Diamond is an excellent insulator and does not conduct electricity.
Both have covalent bonds.
Only Graphite is used as electrodes.
Graphite has a high melting point.
Question
The structures of diamond and graphite are shown.

Which statement about diamond and graphite is correct?
A Diamond and graphite have low melting points.
B Diamond and graphite have mobile electrons.
C Diamond and graphite have layered structures.
D Diamond and graphite contain strong covalent bonds between carbon atoms.
▶️Answer/Explanation
Ans:D
Diamond and graphite have high melting points.
Diamond does not have mobile electrons.
Graphite is a good conductor of electricity. Within each graphene layer, carbon atoms are covalently bonded, but one electron from each carbon atom remains delocalized and is free to move throughout the layers, allowing for electrical conductivity.
Diamond and graphite contain strong covalent bonds between carbon atoms.
Only Graphite has layered structure.
Question
Diamond and graphite have giant covalent structures of carbon atoms. Which statement describes graphite?
A. It has a strong, rigid three-dimensional structure.
B. It has four strong covalent bonds between each carbon atom.
C. It has layers, which can slide over each other.
D. It has no free electrons, so does not conduct electricity.
▶️Answer/Explanation
Ans:
C

Graphite is a good conductor of electricity. Within each graphene layer, carbon atoms are covalently bonded, but one electron from each carbon atom remains delocalized and is free to move throughout the layers, allowing for electrical conductivity.
Graphite is soft and slippery due to the weak forces between the layers. The layers can easily slide past each other, giving graphite its lubricating properties.
Question
Which pair of statements about diamond and graphite is correct?

A. Diamond and graphite are both pure carbon. They are both macromolecules.
B. Diamond and graphite can both be used as electrodes. Graphite is also used as a lubricant.
C. Diamond has covalent bonds. Graphite has ionic bonds.
D. Diamond is hard with a high melting point. Graphite is soft with a low melting point.
▶️Answer/Explanation
Ans:
A
Diamond and graphite are both pure carbon. They are both macromolecules.
Graphite is soft and slippery due to the weak forces between the layers. The layers can easily slide past each other, giving graphite its lubricating properties.
Diamond is an excellent insulator and does not conduct electricity.
Both have covalent bonds.
Only Graphite is used as electrodes.
Both Diamond and Graphite have high melting points.