Question
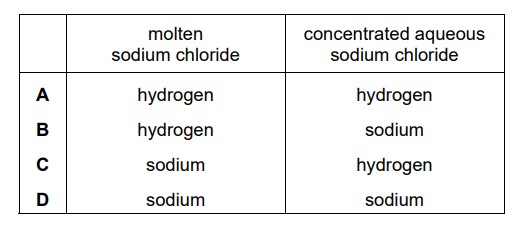
Molten sodium chloride and concentrated aqueous sodium chloride are electrolysed using platinum electrodes. What are the products at the negative electrode (cathode) in each electrolysis?
▶️Answer/Explanation
Ans:
C
In the electrolysis of molten sodium chloride (NaCl), the following reactions occur at the electrodes:
At the negative electrode (cathode): 2Na+ + 2e– → 2Na
Sodium ions (Na+) are reduced to sodium metal (Na) by gaining two electrons.
At the positive electrode (anode): 2Cl– → Cl2 + 2e–
Chloride ions (Cl–) are oxidized to chlorine gas (Cl2) by losing two electrons.
In the electrolysis of concentrated aqueous sodium chloride (NaCl) solution, the presence of water affects the reactions at the electrodes. The water molecules can be oxidized or reduced in addition to the chloride ions. The reactions can be summarized as follows:
At the negative electrode (cathode): 2H2O + 2e– → H2 + 2OH–
Water molecules (H2O) are reduced to hydrogen gas (H2) and hydroxide ions (OH–) by gaining two electrons.
At the positive electrode (anode): 4Cl– → 2Cl2 + 4e–
Chloride ions (Cl–) are oxidized to chlorine gas (Cl2) by losing two electrons.
So, in the electrolysis of concentrated aqueous sodium chloride, hydrogen gas and hydroxide ions are produced at the negative electrode (cathode), while chlorine gas is produced at the positive electrode (anode).
Question
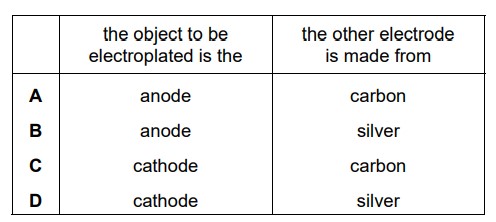
An object is electroplated with silver using an aqueous silver salt as the electrolyte. Which row is correct?
▶️Answer/Explanation
Ans:
D
The object to be electroplated is typically referred to as the “substrate” or “workpiece.” It can be any material that is capable of conducting electricity, such as metal, plastic, or even glass. The purpose of electroplating is to enhance the appearance, provide corrosion resistance, or improve conductivity of the substrate.
In the electroplating setup, the object to be electroplated (substrate) is connected to the negative terminal of the power supply and serves as the cathode.
The anode, on the other hand, is a piece of silver metal or another conductive material that is made of the same metal being used for electroplating, in this case, silver. The anode is connected to the positive terminal of the power supply.
Question
Which process is used to obtain the metal calcium from its ore?
A. electrolysis
B. oxidation with carbon
C. reduction with carbon
D. thermal decomposition
▶️Answer/Explanation
Ans:
A
The metal calcium is obtained from its ore through a process called electrolysis.
Question
Concentrated aqueous sodium chloride is electrolysed using platinum electrodes.
What is the major product formed at each electrode?

▶️Answer/Explanation
Ans:A
In the electrolysis of concentrated aqueous sodium chloride (NaCl) using platinum electrodes, the following reactions occur at the electrodes:
At the negative electrode (cathode): 2H2O + 2e– → H2 + 2OH–
Water molecules (H2O) are reduced to hydrogen gas (H2) and hydroxide ions (OH-) by gaining two electrons. The major product formed at the cathode is hydrogen gas.
At the positive electrode (anode): 4Cl– → 2Cl2 + 4e–
Chloride ions (Cl–) are oxidized to chlorine gas, Cl2 by losing two electrons. The major product formed at the anode is chlorine gas.
Question
Three substances are listed.
1 copper
2 dilute sulfuric acid
3 solid lead(II) bromide
Which substances conduct electricity?
A 1,2 and 3
B 1 and 2 only
C 1 and 3 only
D 2 and 3 only
▶️Answer/Explanation
Ans:B
Solid lead(II) bromide is not a conductor of electricity, it can exhibit conductivity when it is melted or dissolved in a suitable medium.