Question
Which row describes the changes that occur in an endothermic reaction?

▶️Answer/Explanation
Ans:
C
In an endothermic reaction, the system absorbs heat or energy from its surroundings. This results in a decrease in the temperature of the surroundings.
Question
Which change of state is an exothermic process?
A. condensation
B. evaporation
C. melting
D. sublimation
▶️Answer/Explanation
Ans:
A
The change of state from gas to liquid (condensation) is an exothermic process.
When a gas transitions to a liquid, the molecules come closer together and the intermolecular forces between them increase. This results in the release of energy in the form of heat. The heat energy is transferred from the gas to the surroundings, increasing the temperature of the surroundings.
Question
The energy level diagram shows the energy of the reactants and products in a chemical reaction.

Which row correctly describes the energy change and the type of reaction shown?

▶️Answer/Explanation
Ans:B
If the energy of the products is less than the energy of the reactants, it indicates an exothermic reaction.
In an exothermic reaction, the excess energy is released into the surroundings. The reactants have a higher energy level, and as the reaction proceeds, energy is given off in the form of heat or light, resulting in a decrease in the overall energy of the system. The released energy can be observed as an increase in temperature or the production of light.
Question
Sodium nitrate is added to water in a beaker and stirred until it dissolves.
At the end of the experiment, the beaker feels cold.
Which row describes the reaction?

▶️Answer/Explanation
Ans:A
When the beaker feels cold after adding sodium nitrate to water, it indicates that the dissolution of sodium nitrate in water is an endothermic process. Energy is being absorbed from the surroundings, resulting in a decrease in temperature.
Question
Which changes occur when hydrogen is burned in oxygen?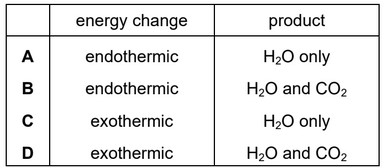
▶️Answer/Explanation
Ans: C
The combustion of hydrogen gas in the presence of oxygen is indeed an exothermic reaction.
When hydrogen gas combines with oxygen, a highly exothermic reaction occurs, releasing a large amount of energy in the form of heat and light and forms water molecules.