Question
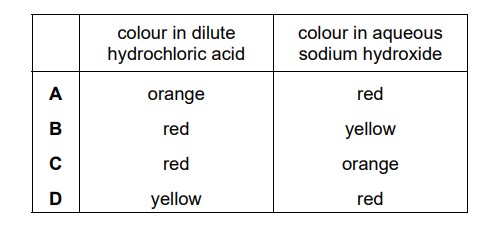
Methyl orange is added to dilute hydrochloric acid and to aqueous sodium hydroxide. What is the colour of the methyl orange in each solution?
▶️Answer/Explanation
Ans:
B
Methyl orange is an acid-base indicator that undergoes a color change in response to changes in pH. In acidic solutions, methyl orange appears red, while in basic solutions, it appears yellow.
When methyl orange is added to dilute hydrochloric acid (HCl), which is an acid, the solution remains acidic. Therefore, methyl orange will exhibit its red color in the presence of the acid.
On the other hand, when methyl orange is added to aqueous sodium hydroxide (NaOH), which is a strong base, the solution becomes basic. In this basic environment, methyl orange will undergo a color change and appear yellow.
Question
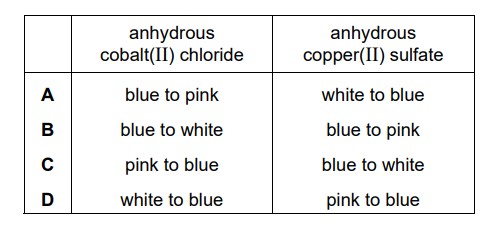
Which row describes the colour changes when water is added to anhydrous cobalt(II) chloride and anhydrous copper(II) sulfate?
▶️Answer/Explanation
Ans:
A
Anhydrous cobalt(II) chloride has a blue color. However, when water is added to it, the compound undergoes hydration and forms a complex compound called cobalt(II) chloride hexahydrate. As a result, the color of the compound changes from blue to pink.
Anhydrous copper(II) sulfate is a white or grayish compound. However, upon the addition of water, it forms a hydrated compound known as copper(II) sulfate pentahydrate. The color of copper(II) sulfate pentahydrate is bright blue.
Question
What is a characteristic of acids?
A Acids turn methyl orange indicator yellow.
B Acids have a high pH value.
C Acids react with ammonium salts to give ammonia gas.
D Acids react with carbonates to produce salts.
▶️Answer/Explanation
Ans:D
When acids react with carbonates, they produce salts, water, and carbon dioxide gas. This type of reaction is known as an acid-carbonate reaction or acid-base reaction.
Question
Ammonium chloride is heated with aqueous sodium hydroxide.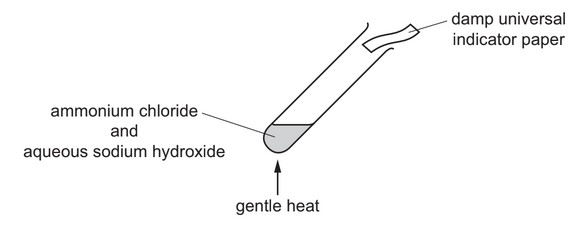
A gas is produced which turns damp universal indicator paper blue.
Which gas has been produced?
A ammonia
B hydrogen
C oxygen
D sulfur dioxide
▶️Answer/Explanation
Ans: A
When ammonium chloride reacts with sodium hydroxide (NaOH), it produces ammonia gas in addition to water and sodium chloride (NaCl).
Question
Some reactions of element M are shown.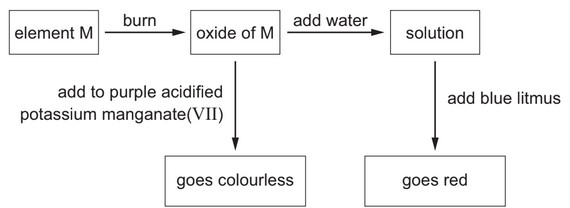
What is element M?
A carbon
B iron
C magnesium
D sulfur
▶️Answer/Explanation
Ans: D
The reaction between sulfur dioxide and acidified potassium permanganate results in a colorless solution due to the conversion of sulfur dioxide to sulfuric acid and the reduction of potassium permanganate.
The addition of water to sulfur dioxide can cause blue litmus paper to turn red due to the acidic nature of the resulting solution i.e. sulfurous acid.