Question
Oxides of nitrogen are given out from car exhausts.
Which row best shows why oxides of nitrogen are unwanted in the atmosphere?

▶️Answer/Explanation
Ans:D
When fuel is burned in an internal combustion engine, such as in cars, nitrogen and oxygen from the air combine under high temperatures to form various oxides of nitrogen, primarily nitrogen monoxide (NO) and nitrogen dioxide (NO2). These gases are collectively referred to as NOx.
Nitrogen dioxide is a particularly harmful component of NOx. It is a reddish-brown gas with a pungent odor. NO2 can cause respiratory problems, irritation of the respiratory system, and contribute to the development or exacerbation of conditions such as asthma and other lung diseases. It can also have negative environmental impacts on plants and ecosystems.
Furthermore, NOx gases contribute to the formation of nitric acid in the atmosphere, which is a corrosive and environmentally harmful acid. Nitric acid can damage buildings, infrastructure, and vegetation when it falls as acid rain.
Question
Four different groups of oxides are shown.
$1 \mathrm{MgO} \mathrm{FeO} \mathrm{CuO}$
$2 \mathrm{CaO} \quad \mathrm{SO}_2 \quad \mathrm{TiO}_2$
$3 \mathrm{PbO} \mathrm{CaO} \quad \mathrm{Cl}_2 \mathrm{O}$
$4 \quad \mathrm{NO}_2 \quad \mathrm{Br}_2 \mathrm{O} \quad \mathrm{P}_2 \mathrm{O}_5$
Which statement about these oxides is correct?
A 1,2 and 3 contain basic oxides only.
B 2, 3 and 4 contain basic oxides only.
C 1 contains basic oxides only and 4 contains acidic oxides only.
D 1 contains acidic oxides only and 4 contains basic oxides only.
▶️Answer/Explanation
Ans:C
Metal oxides are basic hence, 1 contains basic oxides only.
Non metal oxides are acidic, hence 4 contains acidic oxides only.
Question
Phosphorus is an element in Group V of the Periodic Table. It burns in air to form an oxide, which dissolves in water to form a solution with a pH of 1. Which row describes this oxide of phosphorus?

▶️Answer/Explanation
Ans:C
P is a non metal. Non metal oxides are acidic.
Question
Aluminium oxide has the formula $\mathrm{Al}_2 \mathrm{O}_3$.
Which statement about aluminium oxide is correct?
A $2 \mathrm{~g}$ of aluminium atoms are combined with $3 \mathrm{~g}$ of oxygen atoms.
B $2 \mathrm{~g}$ of aluminium atoms are combined with $3 \mathrm{~g}$ of oxygen molecules.
C Aluminium oxide has a relative formula mass of 102 .
D Pure aluminium oxide contains a higher mass of oxygen than of aluminium.
▶️Answer/Explanation
Ans:C
The relative formula mass of aluminium oxide, $\mathrm{Al}_2 \mathrm{O}_3$ can be calculated by summing the atomic masses of all the atoms in the compound.
= (2 × atomic mass of Al) + (3 × atomic mass of O) = (2 × 27) + (3 × 16) = 54 + 48 = 102 g/mol.
Question
Element X forms an oxide, XO, that neutralises sulfuric acid.
Which row describes X and XO?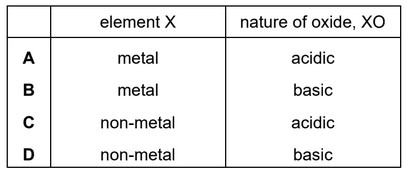
▶️Answer/Explanation
Ans: B
Since it neutralises sulfuric acid, XO is a basic oxide, hence X is a metal.