Question
Which statement about the Periodic Table is correct?
A. Elements with the highest atomic number in each period are metallic.
B. Elements with the lowest group numbers are non-metals.
C. Elements with similar chemical properties are placed in groups.
D. Elements with similar physical properties are placed in periods.
▶️Answer/Explanation
Ans:
C
The periodic table is organized into groups or columns, and elements within the same group share similar chemical properties.
Each group in the periodic table is labeled with a number and often a letter, such as Group 1 (also known as the alkali metals), Group 2 (alkaline earth metals), Group 17 (halogens), and Group 18 (noble gases). These groups contain elements that exhibit similar behaviors and react in comparable ways.
Question
The positions of four elements in the Periodic Table are shown.
Which element is a gas that displaces iodine from sodium iodide?

▶️Answer/Explanation
Ans:C
Chlorine (Cl) can displace iodine (I) from sodium iodide (NaI) through a redox reaction. This reaction occurs because chlorine is more electronegative than iodine, meaning it has a greater affinity for electrons.
Question
The elements in Period 3 of the Periodic Table are shown.

Which statements about the elements in Period 3 are correct?
$1 \mathrm{Na}, \mathrm{Mg}$ and $\mathrm{Al}$ are metals.
$2 \mathrm{~S}, \mathrm{Cl}$ and $\mathrm{Ar}$ are non-metals.
$3 \mathrm{Si}, \mathrm{P}$ and $\mathrm{S}$ are metals.
A 1 and 2 only
B 1 and 3 only
C 2 and 3 only
D 1,2 and 3
▶️Answer/Explanation
Ans:A
Silicon is a metalloid, which means it has properties that are intermediate between those of metals and nonmetals.
Phosphorus, on the other hand, is a nonmetal.
Question
Part of the Periodic Table is shown.
Which element forms an acidic oxide?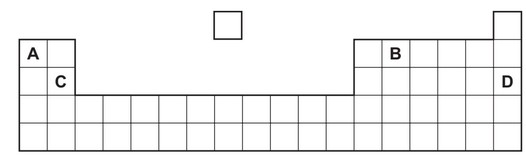
▶️Answer/Explanation
Ans: B
B is a non metal. B is carbon and carbon is a nonmetal and can form acidic oxides, such as carbon dioxide, which can further dissolve in water to produce carbonic acid.
Question
Which statements describe the Periodic Table?
1 The elements are arranged in order of their nucleon number.
2 The elements are arranged in order of their proton number.
3 It is used to predict the properties of elements.
A 1 and 3 B 1 only C 2 and 3 D 2 only
▶️Answer/Explanation
Ans: C
The elements are arranged in order of their proton number, also called as atomic number.
Periodic table is used to predict the properties of elements.